Table 7.
The newly designed xanthone derivatives and their predicted cytotoxic activities calculated by using the best QSAR model
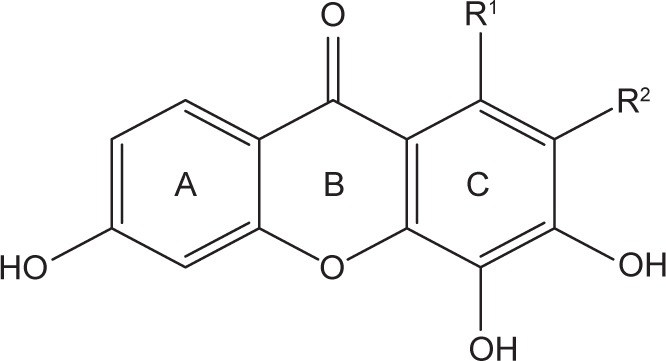
| ||||
|---|---|---|---|---|
| Compound | R1 | R2 | IC50 (µg/mL) | IC50 (µM) |
| 11 | OH | H | 0.013 | 0.049 |
| 12 | OCH3 | H | 0.016 | 0.058 |
| 13 | NH2 | H | 1.662 | 6.413 |
| 14 | N(CH3)2 | H | 0.276 | 0.960 |
| 15 | H | SH | 0.038 | 0.136 |
| 16 | SH | SH | 0.091 | 0.299 |
| 17 | NH2 | SH | 0.134 | 0.460 |
| 18 | OCHCH2 | H | 0.100 | 0.349 |
| 19 | OCH2CH2CH3 | H | 0.029 | 0.095 |
| 20 | C(CH3)3 | H | 3.586 | 11.940 |
| 21 | OCOCH3 | H | 0.011 | 0.035 |
| 22 | NHCOCH3 | H | 0.006 | 0.021 |
| 23 | N(C(CH3)3)2 | H | 5.498 | 14.188 |
| 24 | CHC=C(CH3)2 | H | 5.404 | 18.117 |
| 25 | C(CH2CH3)3 | H | 0.705 | 2.059 |
Abbreviations: IC50, inhibitory concentration 50%; QSAR, quantitative structure–activity relationship.
