Figure 1.
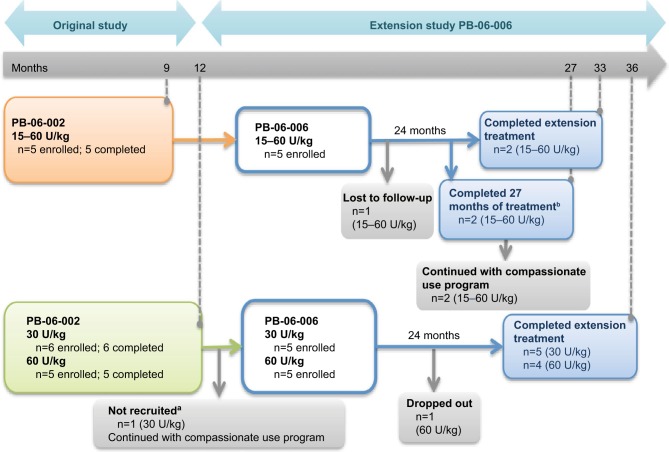
PB-06-002 was a switchover study from imiglucerase to taliglucerase alfa.
Notes: aPatient had GD Type 3c and cardiac involvement requiring intervention due to decompensation. bPatients did not reach 33 total months of treatment due to early closure of study. Patients were either maintained on the same dose as received on imiglucerase, or if they were on <60 U/kg of imiglucerase, dose could be increased based on medical condition and at the investigator’s discretion. The five pediatric patients who completed this trial were enrolled in the PB-06-006 trial. Of these, one was lost to follow-up, two completed the 24 months duration of the extension trial, and two completed only 18 months of the extension trial due to early closure of the study; however, both patients continued to receive therapy through the compassionate use program. PB-06-005 involved the treatment of naïve pediatric patients who received either 30 or 60 U/kg of taliglucerase alfa for 12 months. Out of the 11 patients who completed the PB-06-005 trial, 10 were enrolled in the PB-06-006 extension trial. One patient had Type 3c GD and cardiac involvement requiring intervention due to decompensation and was not recruited into the extension phase, one patient dropped out from the trial, and nine patients completed the PB-06-006 extension trial. Out of the 15 patients enrolled in the PB-06006 extension trial, 11 completed. Reprinted from Blood Cells Mol Dis. Vol S1079–9796(16). Zimran A, Gonzalez-Rodriguez DE, Abrahamov A, et al. Long-term safety and efficacy of taliglucerase alfa in pediatric Gaucher disease patients who were treatment-naïve or previously treated with imiglucerase. Pages 30221–30222. Copyright 2016, with permission from Elsevier.25
Abbreviation: GD, Gaucher disease.