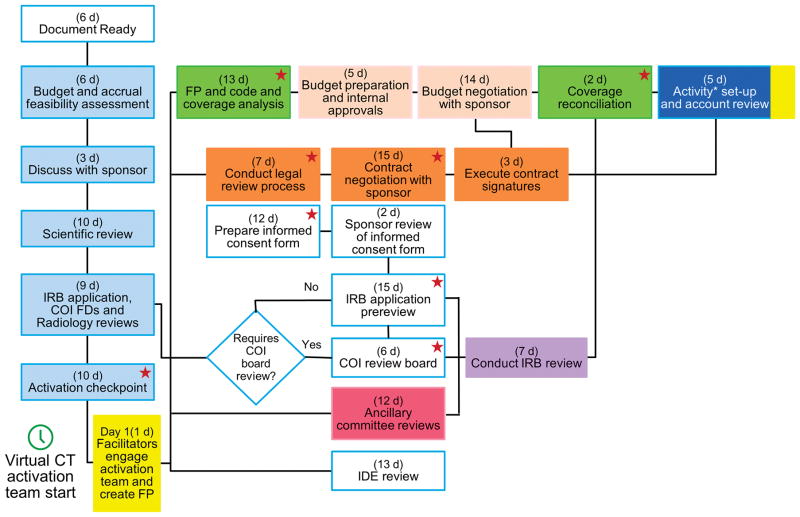
Figure 1.
Transformed Process Flow for Transforming the Activation of Clinical Trials at a Single Site. In each box, the exhibits represent maximum duration (business days) assigned to each process step. This exhibit represents the flow for part 1 studies. For part 2 studies, the design was modified slightly on the basis of the plan-do-study-act process. All activities affecting the consent form are designated with the star icon. Asterisk indicates 39 business days from creation of FP to financial activation. The colors corresponds to colors for corresponding steps in Supplementary Table 1. COI indicates conflict of interest; CT, clinical trial; FD, financial disclosure; FP, funding proposal; IDE, Investigational Device Exemption; IRB, institutional review board.