Abstract
Cardamom is a strong antioxidant plant, so it is called the queen of spices. In the present study, we explored the potentials of cardamom on developmental, learning ability and biochemical parameters of mice offspring. Thirty pregnant mice were allocated to three groups of ten animals in each. Groups Π and Ш received pilsbury's Diet containing 10 and 20% of cardamom (w/w) respectively, whereas Group I used as control. Cardomom was administered from the first day of pregnancy and was continued until post-natal day 15 (PD 15) and thereafter the mothers were switched to plain pilsbury's Diet. During the weaning period, three pups in each litter were color marked from the others, and were subjected to various tests (Physical assessment such body weight and eye opening and hair appearance; the neuromaturation of reflexes like righting, rotating, and cliff avoidance reflexes; learning ability and memory retention; estimation of monoamines neurotransmitters like dopamine and serotonin, non-enzymatic oxidative stress such as TBARS and GSH in forebrain at different ages of pups). The results indicated that the body weight gain was declining significantly. Hair appearance and eyes opening were delayed significantly. Righting, rotating, and cliff avoidance reflexes were delayed in treated animals. Exposure to cardamom led to enhance learning and memory retention as compared to control. Monoamines (DA, 5-HT) and GSH were elevated, whereas TBARS was inhibited significantly. In conclusion, perinatal cardamom exposure enhanced learning and memory as compared to control. Cardamom and its benefit compounds were transported via placenta or/and milk during lactation. Cardamom needs more researches to investigate its benefits on other kinds of behavior.
Keywords: Cardamom, Elettaria cardamomum, Learning, Mice offspring
1. Introduction
The most worldwide famous traditional aromatic plant Cardamom (Elettaria cardamomum Maton) is grown and cultivated in some Asian countries such as Sri Lanka, India, Nepal, Indonesia, Guatemala and Tanzania, and is belonging to Zingiberaceae (Garg et al., 2016). In India, it is mainly known as the Queen of spices whereas in gulf countries such as Saudi Arabia, Kuwait, United Arab Emirates, Iraque, Iran and other terrestrial regions, it is known as “Hel” (Nirmala, 2010). The Cardamom has been known since about 3000 years BC. The scientific name of Cardamom, Elettaria cardamomum, came from Tamil language (The popular South Indian languages). The colloquial word Elettari is referring to the cardamom seeds in Tamil language (Mahindru, 1982). The dried seeds are used as a flavoring agent in coffee, cakes and curries. In all regions of Saudi Arabia, the Cardamom seeds were used widely in Arabian coffee, and as an flavoring ingredient in certain teas. Some people also chew the seeds to freshen breath (Ajarem and Ahmad, 1991). Traditionally, it has been reported that the different parts of E. cardamomum, such as leaves, seeds, bark, root and flowers were applied for the treatment of various intestine related disorders such as stomachic, retentive, resolvent, antiemetic, digestive constipation, hypertension, asthma, diarrhea, colic, dyspepsia, epilepsy and carminative (Das et al., 2012).
Recent advanced inventions in the ethano-pharmacology and phytochemical characterization of this plant guided to identify major metabolites such as alpha pinene (1.5%), beta-pinene (0.2%), sabinene (2.8%), myrcene (1.6%), alpha phellandrene (0.2%), limonene (11.6%), 1,8, cineole (36.3%), terpinene (0.7%), cymene (0.1%), terpenolene (0.5%), linalool (3%) and other components with various biological applications such as antioxidant, anticancer, anti-inflammatory, antibacterial, antifungal, antiproliferative, antidiabetic, and antiviral activities respectively (Sarkar, 2011). Also, few reports claimed that the major components belonged to flavanoids, alkaloids, terpernoids, anthocyanins and other phenolic compounds recovered from E. cardamomum were used for the treatment of cardiovascular, digestive, pulmonary, kidney associated and lung associated disorders, liver disorders and other stress associated diseases (Vaidya and Rathod, 2014). Additionally, the active metabolites from this plant were recognized for the increased modulation of macrophages in the immunological related diseases and protect the human immunological activating cells in the human resulted from various heavy metal hazards (Abdelkader et al., 2015). Some studies have indicated that cardamom could be caused allergic reactions and can interfere with medications, such as liver inflammatory and gallstones drugs (Sengottuvlu, 2011, Aboelnaga, 2015). Another study indicated that cardamom affects the social behavior of the mice offspring, where the body weight decreased and delayed of eyes opening. Social investigations were increased in males, attack, a number of attack times, the beginning of the threat and the time of the attack were declined (Ajarem and Ahmad, 1991). Khan et al. (2011) identified phytochemical constituents such as alkaloids, saponins, flavonoids, tannins and sterols from the seeds of cardamom and determined the carbachol-mediated bronchoconstriction in rats. Khan et al. (2011) claimed that the administration of the crude extracts blocked the Ca++ channel in rats. Alternatively, Hamzaa and Osman, 2012 characterized the phenolic compounds such as pyrogallol acid, gallic acids, protocatechouic acid, catechin and caffeic acid and essential oils such as germaniol, α-pinene, sabimene, limonene, 1,8 cineol, γ-terpinene, terpinolene, linaiool, terpineol, octyl acetate, linalyl acetate, terpenyl acetate and neryl acetate from the seeds and investigated the potential application related to the antioxidant and reduce the oxidative stress induced by γ-irradiation exposure. Goyal et al. (2015) reported that the chemical components of cardamom have leading activity against Myocardial Infarction by restoring endogenous antioxidants. Considering all the positive effects, the present study elucidates the effects of cardamom on the development, behavior and biochemical parameters of mice offspring.
2. Materials and methods
2.1. Animals
Three females to one male Swiss–Webster strain mice (10–12 weeks old) were grown and maintained in separate opaque plastic sheltering cageswith dimensions of measuring 30 × 12 × 11 cm, in the animal health house monitored in the department of Zoology, King Saud University, Saudi Arabia. The external conditions were maintained as (with white lights on from 22.30 to 10.30 h local time) and with the temperature (maintained with the range of 18 and 22 °C) respectively. On the first day of the pregnancy the male mice were transferred to separate cultivation cages whereas the females were examined to the experimental treatments.
2.2. Cardamom administration and experimental design
Unbleached and dry capsules (Seeds and pods) of cardamom were purchased from the local market for the present study. Cardamom and Pilsbury's Diet were mixed in such ratio that it provided doses of 10 and 20% of cardamom (w/w). The mixtures were minced in a grinder before adding distilled water to it. The dough was cut into plates similar to pilsbury's Diet in size and was dried at room temperature. These plates formed the only food for treated groups, whereas plain pilsbury's Diet was given to the control group.
Thirty pregnant mice (Vaginal plug appearance) were allocated to three groups of ten animals in etch. Groups Π and Ш received pilsbury's Diet containing 10 and 20% of cardamom (w/w) respectively, whereas Group I used as control. Food and water were available to all groups ad libitum except during the behavioral trials. On the day of pregnancy (Vaginal plug appearance), all animals were divided into two subgroups as flowing:
-
1.
1: Group I was subjected to Cognitive behavioral in shuttle box and water maze tests.
-
2.
2: Group II was subjected to biochemical analysis, such as the levels of dopamine (DA) and serotonin (5-HT); non enzymatic oxidative stress (OS) indices like thiobarbituric acid-reactive substances (TBARS) and total reduced glutathione (GSH) in the brain.
Treatment started from day one of pregnancy and was continued until post-natal day 15 (PD 15) and thereafter the mothers were switched to plain pilsbury's Diet. The pups of the identical experimental group were culled to only eight per dam on the post-natal day1 (PD 1) after birth and were left with their mothers until PD 22. The pups accommodated in each boxes were marked separately to distinguish during the weaning period. These marked pups were transferred for the analysis of the behavioral studies under very low intensity lights. The impact of the pups especially the changes in their behavioral attitudes were recorded every other day and performed till the 21 days. The results were recorded in three different coolers marked for the statistical analysis. The collected data were noted for the analysis of the sensory motor coordination reflexes. In addition, the morphological development of the different color pups was also monitored.
2.3. Physical assessment during weaning period
Various physical analysis measurements such as the differences in the body weight and nature of the hair present in the body of the offspring were evaluated from the first day of the birth to the 21st day. All the recorded details were carefully noted for the final confirmation. During the study, body weight of the pups was checked alternative days of the incubation time, whereas, the nature of the hair is analyzed from the first day of the hair appearance in the pups. The body weight and the hair are the major morphological indicators in the developmental stages.
2.4. Neuromotor maturation assessment during weaning period
The neuromotor maturation of the developing reflexes in the developing offspring were measured on every alternative day starting from day 1 by performing various tests such as righting, cliff avoidance and rotating reflexes respectively for 21 days of the weaning periods. Briefly, for righting reflex was measured by the difference in time taken by the pup to turn the original places for the individual paw. Similarly, all the four paws kept on the substrate was measured. For this test, maximum 120 s were kept as the limited time. However, for analyzing the cliff avoidance, the pups were kept in the edge part and from there the time taken for the pups to realize the edge and moving back to the original spots were measured. For this test, maximum 120 s were kept as the limited time. For, rotating reflex, the pups were kept in the angle surface and the time taken to rotate 30 degree angle was quickly recorded. Also, the pups were kept under the surface with downward position of the head. The required time to rotate the head to the original position and look towards the upper side was measured. For this test, maximum 120 s were kept as the limited time.
2.5. Cognitive behavioral assessment during post-weaning period
The following tests were evaluated in the same cohort of male offspring (bearing in mind to include representatives of each litter) in all the behavioral tests.
2.5.1. Active avoidance responses
For measuring this response, completely automated reflex conditioner “shuttle box” was used. The operating conditions were followed as per the instruction of the device. Briefly, starting the experiment each pupwas adapted to stay in the box without any stimulus for 120 s. For the initiating the experiment, the device was started with the duration of six seconds with (21 W) and buzzer for (670 Hz and 70 dB). The detailed methodology was previously published Abu-Taweel et al. (2012). During the experiment, the change in the stimulus was measured by the pups with running time to the separate chamber in the device with in the 5 s time. This experiment was conducted 50 times for the accurate measurement for each animal. The spontaneous migration of the mouse to the other compartment between trials was also assessed by measuring the number of crossings between the chambers when no shock was present during UCS and CS (inter-trial crossing). The recorder unit of the automated shuttle box continuously recorded these parameters during the whole experimental period (50 trials) of each animal.
2.5.2. Morris water-maze test
Morris water-maze test was used for the evaluation of cognitive functions in mice (Rutten et al., 2002, Tariq et al., 2008). The visual–spatial memory of the mice was recorded in the test. The detailed methodology for the determination and handling the experiment is available in the previously published research article (Abu-Taweel, 2012). The instrument contained the water protected in the galvanized water storing tank. For performing this experiment, all pups were allowed to swim for 1 min in the initial day without any holdings. And, in the second to fifth day, the pups were trained to swim with submerged platform. The latency from immersion into the pool to escape onto the hidden platform (maximum duration of trial 120 s) was recorded. The unsuccessful trials were noted in the percentage of the failure in each day of the swimming of the pups. In addition, the hippocampal-dependent spatial reference memory was noted from the pups by following the methodology of (Jeltsch et al., 2001, Spiers et al., 2001).
2.6. Biochemical studies
For performing the various biochemical tests, ten numbers of offsprings with different age groups such as (7 PD, 14 PD, 21 PD, 30 PD and 36 PD) from each group of the study were selected and sacrificed. After that, neurotransmitters and some enzymatic and non-enzymatic oxidative stress indices of the offsprings were analyzed from the fore-brain. From the brain, cerebral areas with hippocampus and striatum regions were carefully collected and immediately transferred into the sterile tubes and lyophilized at −70 °C for the routine laboratory analysis.
2.6.1. Determination of monoamines
The methodology followed by Patrick et al., 1991, was slightly modified for the estimation of monoamine neurotransmitters dopamine (DA) and serotonin or 5-hydroxytryptamine (5-HT). In details, ten percentage of the lyophilized brain was mixed with buffer solution contains 0.1 M HClO4 and 0.05% EDTA for 10 s, after that the slurry was centrifuged at 17,000 rpm at 4 °C for 5 min. The collected cell free supernatant was used for the quantification of the individual monoamines by HPLC. The elution buffer contains citric acid monohydrate (32 mM), disodium hydrogen orthophosphate (12.5 mM), methanol (7%), octane sulfonic acid (1 mM) and EDTA (0.05 mM). C18 column was used for the separation of the individual monoamines with the flow rate of 1.2 ml/min. For the quantification, 20 μl of the cell free supernatant was injected in the HPLC. The amount of DA and 5-HT was calculated based on the calibration curve. The final results were noted as ng/mg tissue weight.
2.6.2. Determination of non-enzymatic oxidative stress indices
2.6.2.1. Lipid peroxides
Thiobarbituric acid-reactive substances (TBARS) methodology was followed for the estimation of lipid peroxides (LP). The enzyme was measure by spectrophotometrically as reported by Ohkawa et al. (1979). An extinction coefficient of 1.56 × 105 m−1 cm−1 was used for the calculation of the lipid peroxides. The final results were noted as ng/mg tissue weight.
2.6.2.2. Glutathione
The detailed methodology followed by Mangino et al. (1991) was executed for the determination of the reduced glutathione (GSH) in the brain cells. The color change in the reaction mixcture was recorded for the GSH level. The specific activity is expressed into umol/g tissue weight.
2.7. Statistical analysis
One-way analysis of variance (ANOVA) was followed for the determination of the significance and the variations in the experimental groups. For the significance test, Student-Newman-Keuls multiple comparison test was also followed. The levels of significance were defined at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001.
3. Results
3.1. Physical assessment during weaning period
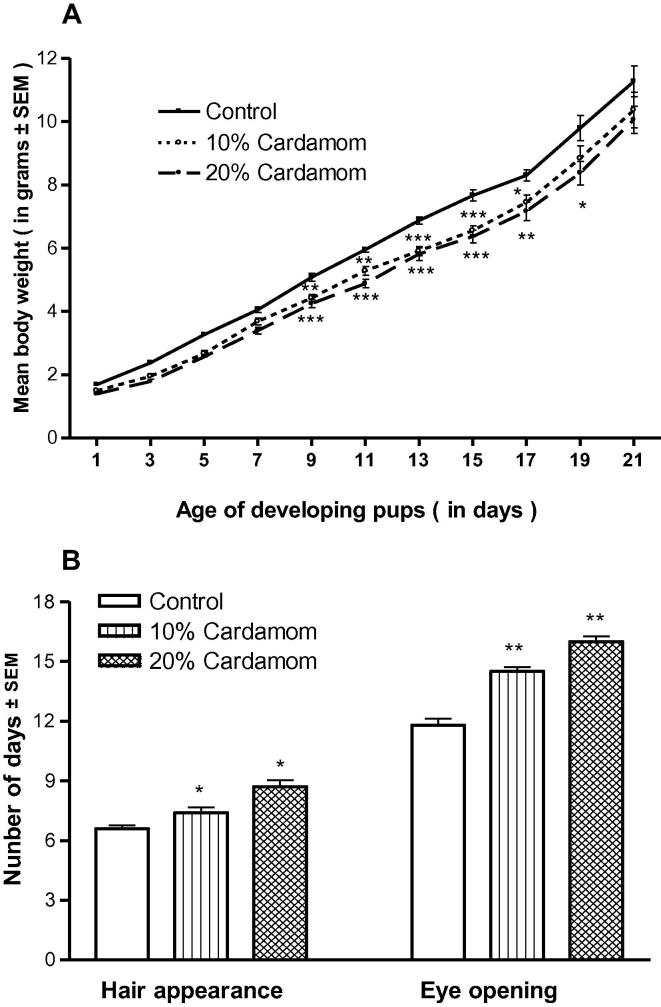
Fig. 1A showed that cardamom perinatal exposure led to reduce body weight gain of treated offspring. The reduction was significant (p < 0.001) in the days (9, 11, 13), and was (p < 0.01) in day 17, and was (p < 0.05) in day 19 in high dose treated offspring. The effect of low dose of cardamom was (p < 0.01) in the days (9, 11), and was (p < 0.001) in the days (13, 15), and was (p < 0.05) in day 17 as compared to control group.
Fig. 1.
(A and B) Effect of perinatal cardamom doses (10 and 20%) exposure on the dose-dependent body weight gain (A) and body hair appearance and eye opening in mouse pups during the weaning (lactation) period. (*, ** and ***) Represent statistically significant (P < 0.05, P < 0.01 and P < 0.001respectively) from the control group; see text.
Hair appearance was delayed significantly (p < 0.05) in perinatal cardamom exposed offspring. The eyes opening were delayed, also, as (p < 0.01) compared to control offspring (Fig. 1B).
3.2. Neuromotor maturation assessment during weaning period
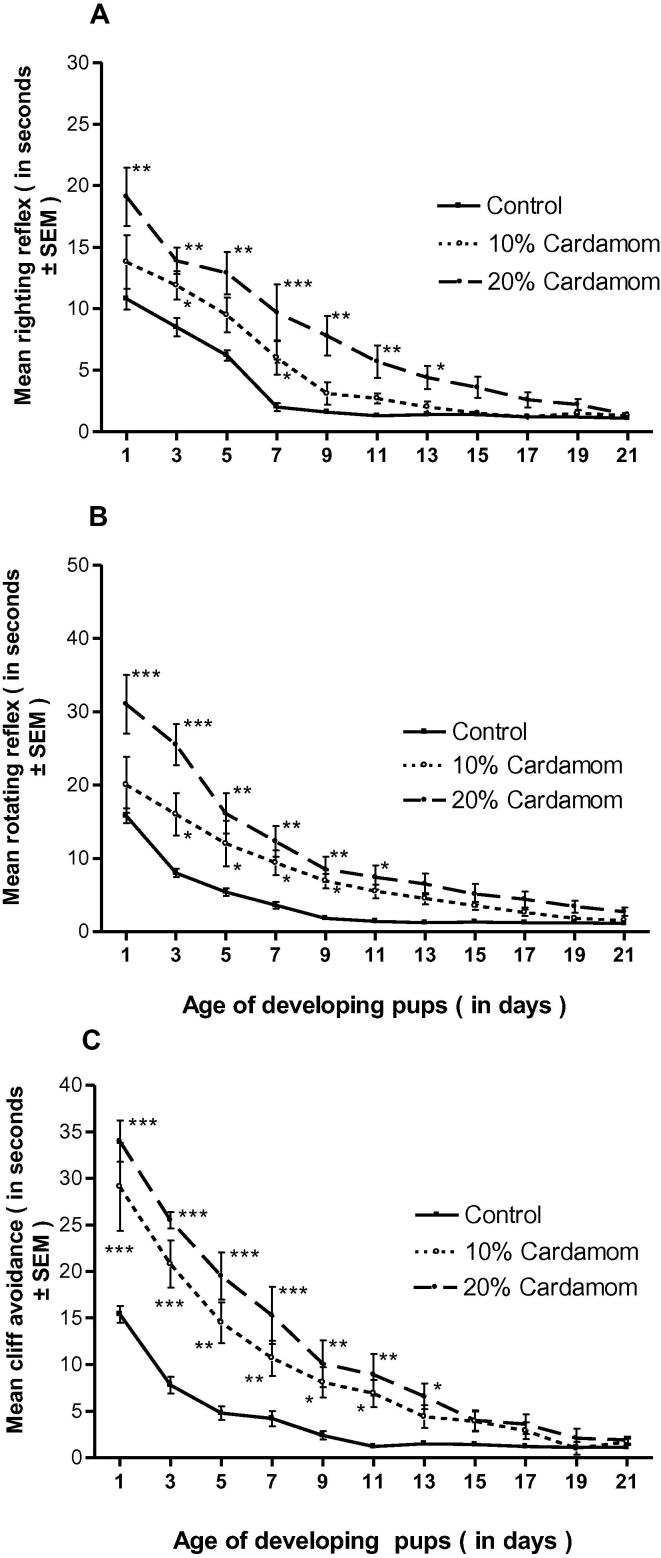
Results indicated that the neuromotor maturation assessment of the pups were comparatively significant and the significance were suppressed with respect to the doses during the weaning periods (Fig. 2A–C).
Fig. 2.
(A–C) Effect of perinatal cardamom doses (10 and 20%) exposure on the dose-dependent righting reflex (A), rotating reflex (B) and cliff avoidance (C) of mouse pups during the weaning (lactation) period. (*, ** and ***) Represent statistically significant (P < 0.05, P < 0.01 and P < 0.001respectively) from the control group.
3.3. Cognitive behavioral studies
3.3.1. Active avoidance test
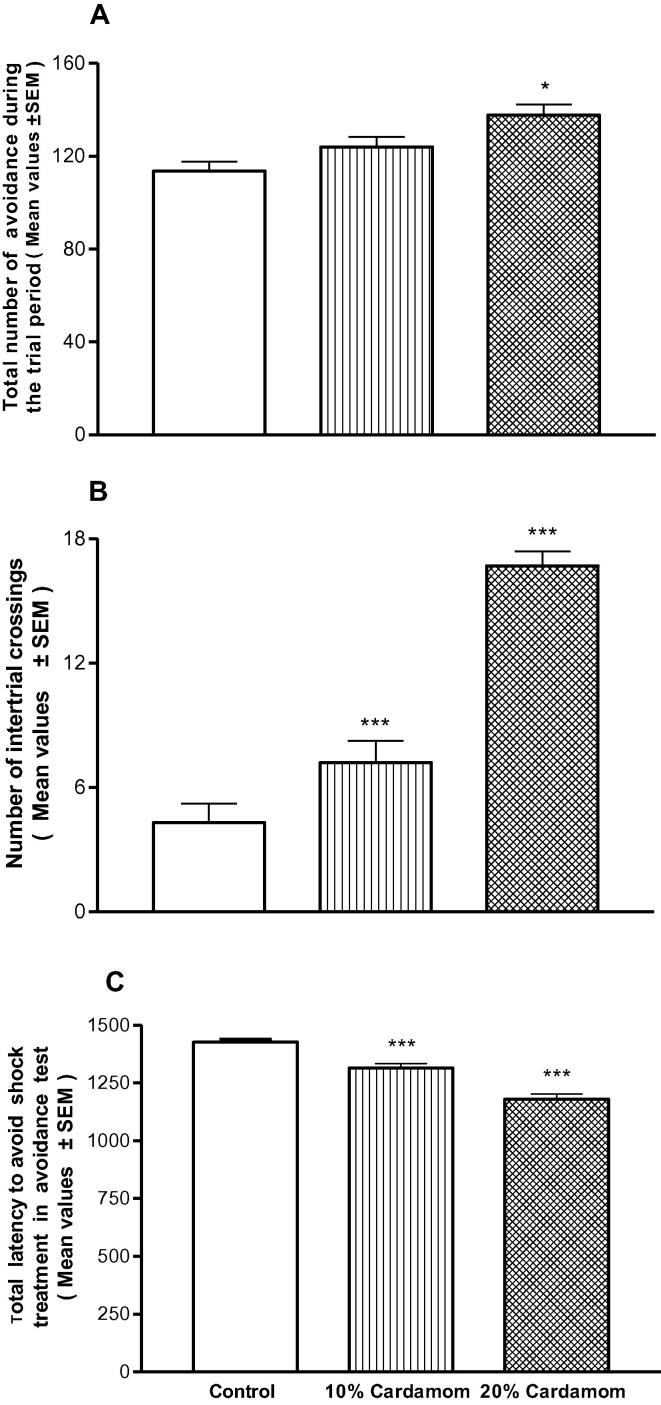
The results indicated that the offspring (PD25) were depending to the concentration of the cardamom samples. It is also confirmed that the increases in the number of the avoidances in the experimental conditions were concentration dependant (Fig. 3A). The spontaneous migration of the mouse was also related to the concentration of the samples and increase in the number of inter-trial crossings as compared to the controls (Fig. 3B). Interestingly, the results confirmed that the animals showed to cardamom were high learners in a dose-dependent manner and took significant time in avoiding the shock treatment as compared to the controls (Fig. 3C).
Fig. 3.
(A–C) Effect of perinatal cardamom doses (10 and 20%) exposure on the dose-dependent mean performance value of the mice offspring at the age of 25 days postnatal in the active avoidance task.
3.3.2. Morris water-maze task
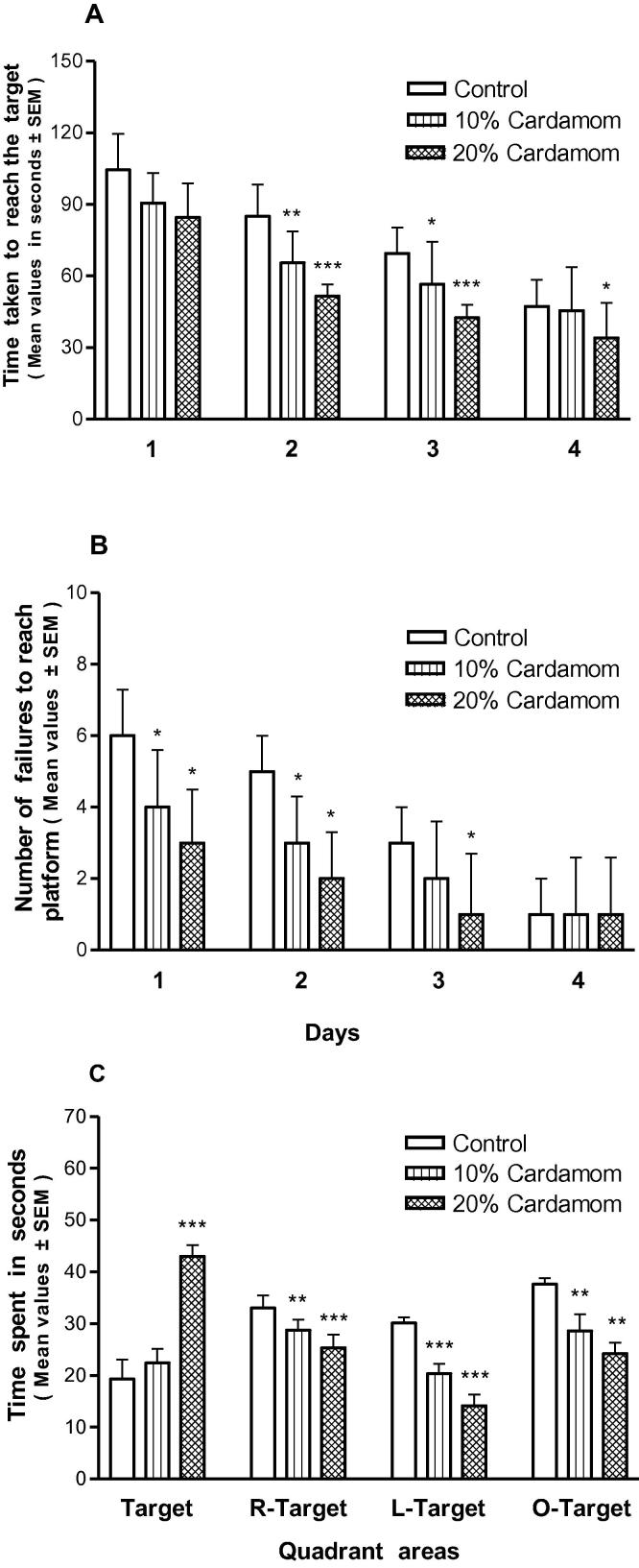
The results of Morris water-maze task revealed that the cardamom treatment, exhibited shorter escape latencies (p < 0.05, p < 0.01, p < 0.001; Fig. 4A), whereas, the other tested groups gradually showed the significant improvement. The rate of the unsuccessful trails to enter the platform was comparatively lesser and the results were significantly strong (p < 0.05; Fig. 4B). The probe trial experiment authenticated that the cardamom exposed offspring spent more time in the other three quadrants than the target (platform) (p < 0.001; Fig. 4C).
Fig. 4.
(A–C) Performance in Morris water-maze of mice offspring at the age of 30 days postnatal whose mothers were exposed perinatally to cardamom doses of (10 and 20%) in a dose-dependent manner. A: Hidden platform (y-axis) on each testing day (x-axis). B – Shows the number of failures or unsuccessful trials (y-axis) on each testing day (x-axis). C – Shows the outcome of the probe test performance. (*, ** and ***) Represent statistically significant (P < 0.05, P < 0.01 and P < 0.001respectively) from the control group.
3.4. Biochemical studies
3.4.1. Levels of monoamines in forebrain
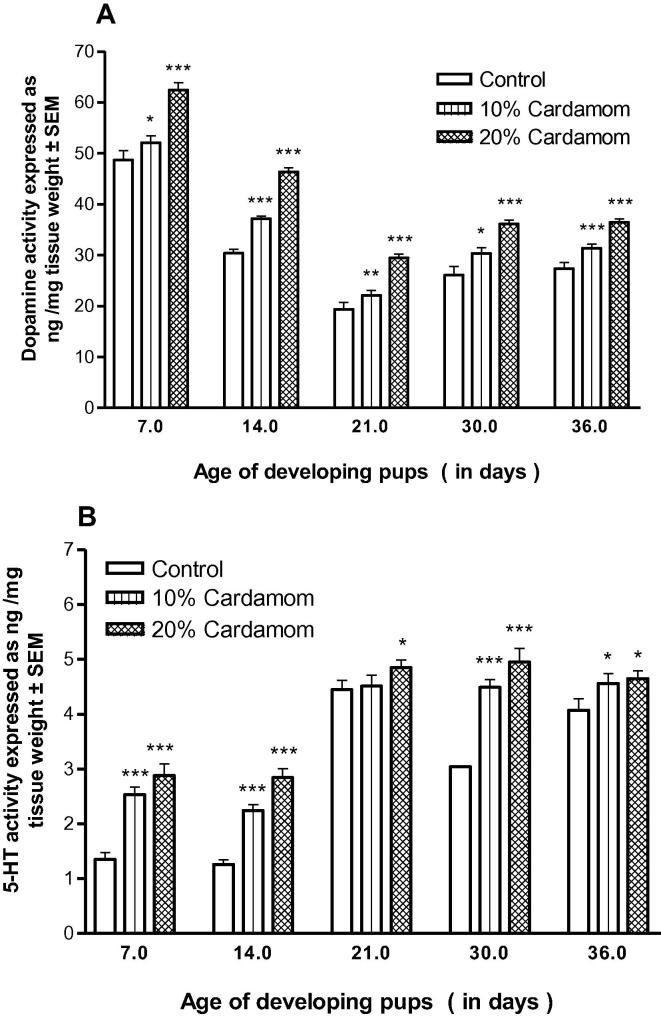
The level of DA and 5HT after treatment was displayed in Fig. 5A and B. The results confirmed that after the treatment the content of the monoamines does dependent and the amounts were comparatively significant (p < 0.001).
Fig. 5.
(A and B) Effect of perinatal cardamom doses (10 and 20%) exposure on the dose-dependent mean levels of dopamine (A) and 5-HT (5-hydroxy-tryptamine or serotonin) (B).
3.4.2. Levels of non-enzymatic oxidative stress indices
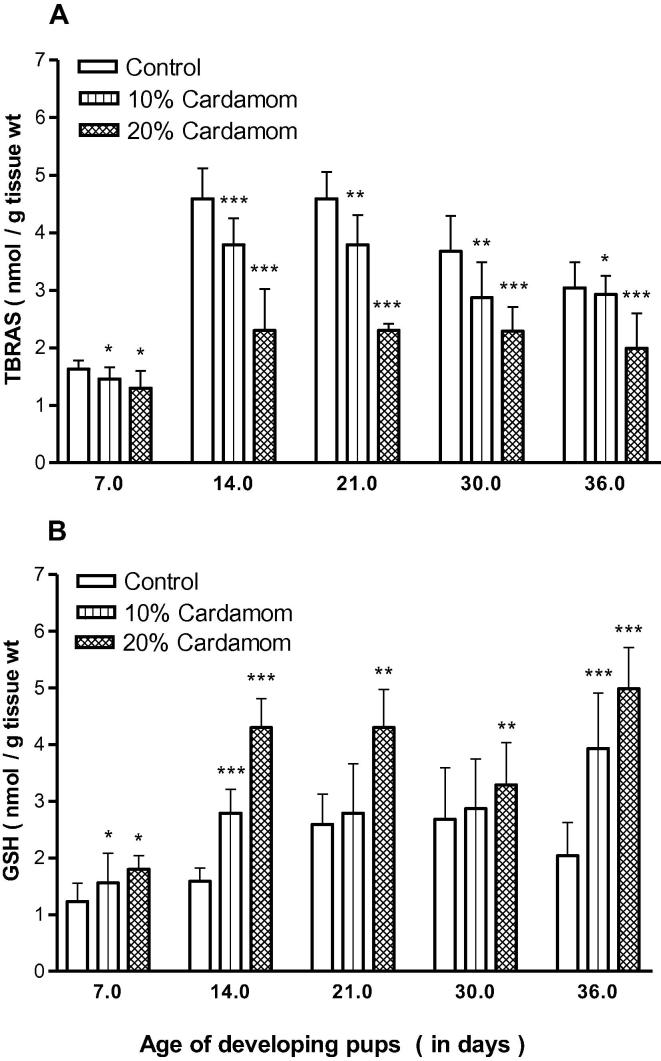
LP determined as TBARS were found to be inhibited significantly (p < 0.001) due to perinatal cardamom exposure in the developing forebrain of the offspring throughout the postnatal development period (PD7, PD14 and PD21) and even at adolescent ages PD30 and PD36 in a dose-dependent manner (Fig. 6A). On the contrary, reduced glutathione (GSH) level remained elevated significant (p < 0.001) at all developmental ages in a dose-dependent manner (Fig. 6B).
Fig. 6.
(A and B) Effect of perinatal cardamom doses (10 and 20%) exposure on dose-dependent mean levels of non-enzymatic oxidative stress indices like (A) lipid peroxidation content (TBARS), and (B) total glutathione (GSH) level, in the forebrain of the offspring at various postnatal developing ages (x-axis).
4. Discussion
The routine uses of spices in our daily food have significant influences in the behavioral stages and physical maturation of the body. It is interesting that the applications of phytochemical constituents from the medicinal plants to heal the disorders related to central nervous system such as depression, psychosis and epilepsy, is attracted worldwide. Especially, the spices commonly used in the daily life have been contributing significant role in the human behavioral and sensor developments. Considering the importance of the spices, the present study investigated the effect of cardamom on the development, behavior and biochemical parameters of mice offspring. The results of the present study confirmed that, the female mice treated with different doses of cardamom revealed comparatively better progress in physical maturation, motor reflex development at weaning age. Furthermore, these pups at early post weaning and adolescent ages showed an improvement in active avoidance and cognitive responses and in the levels of neurotransmitters and non-enzymatic oxidative stress indices in the brain. The result obtained from the present study is closely similar to the report of Ajarem and Ahmad (1991) stating that the exposure of the cardamom gained the body weight, slight delay in the eye opening, delay in the hair fuzz appearance. Also, the present results were disagreed with the report of Patrick et al. (1991) which claiming that the cardamom administration reveals the retardation of growth in mice. The significant decrease in the body weight of treated pups during the weaning period may be due to the presence of cardamom in the milk of the lactating females. Few reports in the earlier stages confirmed that the administration of the significant amount of cardomon guided to enhance the body weight in the pregnancy periods which directly influence the body weight of offsprings (Ordy et al., 1966). Coyle et al. (1976) reported that any drug- induced changes in the mother's milk production around the time of parturition might influence the behavior of offspring. In our results, it was observed that exposure of mothers to cardamom during perinatal period induces behavioral changes in their offspring which lasts for a long time. Several reports authenticated the early exposure of pregnant mice to a wide range of compounds with changes in the subsequent behavior of their offspring (Abu-Taweel, 2012, Abu-Taweel, 2017, Abu-Taweel et al., 2012).
Learning is a change in behavior based on the past experience for the stimulus. There are two types of learning, namely associative and non-associative. Memory is the ability of the organism to register, retain and recall the information at a later date. The different types of memory are short-term and long-term (Setty et al., 2013). In the current study, perinatal exposure to cardamom led to enhance learning and memory retention as compared to control. In Shuttle box tests, the number of times to avoid shocks and a number of crossings were increased, while the total time of trials was decreased as compared to control. In the water maze test, the time which taken to reach the target platform was shorter in treated than control animals, and the failures were decreased in treated animals. In probe test, the treated animals were taken more time in the target area as compared to control animals. Our results are in agreement with the studies (Kaur et al., 2008, Setty et al., 2013).
Cardamom is a strongly antioxidant plant (Dimitrios, 2006, Carvalho et al., 2011, Ghosh and Das, 2014, Przygodzka et al., 2014, Shahidi and Ambigaipalan, 2015, Garg et al., 2016). Asha Davy et al. (2012) pointed that total phenolics, flavonoids and proanthocyanidins were about 15mg GAE/g, 3.59 mg QE/g and 2.84 mg CE/g respectively in cardamom. Cardamom consists of cardamonin or chalcones which are a particular subclass of flavonoids (Tavares et al., 2013, Ghosh and Das, 2014, Shahidi and Ambigaipalan, 2015).
Reactive oxygen produced from various metabolic pathways in the tissues damage the cells by binding to the cell components such as carbohydrates, protein and DNA. There reactive oxygen also involved in atmospheric chemistry, combustion, biochemistry, polymerization, and human physiology (Campana et al., 2004). Therefore, to protect the cells from the reactive oxygen species, antioxidant compounds involved in defense the system by hydrogen donation, electron donation, binding lipid to the components (Shahidi and Ambigaipalan, 2015). Antioxidants recovered from the medicinal plants and difference enzymes derived from various biological sources such as catalase, peroxidases and superoxide dismutase play an important role in minimizing the speed of the oxidation of other compounds (Angelo, 2009). Reports confirmed that the phenolic compounds present in the cardamom revealed the antioxidant activities, also the phenolic compounds stimulated the memory ability. On the other hand, epigallocatechin-3-gallate recovered from the cardamom showed promising neuroprotective activity and revealed activity for the treatment of Parkinson's disease. Kaur et al. (2008) reported that the phenolic compounds epigallocatechin-3-gallate obtained from the cardamom seeds enhanced the protein kinase C in the outer membrane of the mice hippocampus regions. Forster et al. (1996) convinced that the oxidative damage of the free radicals in the cerebral cortex and hippocampus leads to the significant problem to the cognitive functions and thereby affects the brain senescence. Recently, Kaur et al. (2008) confirmed that the drinking green tea supplemented with cardamom decreases the enzymatic conversion of glutathione peroxidase, superoxide dismutase, and lipid peroxidation products and increased the enzymatic reaction of catalase and glutathione reductase. The antioxidant property of cardamom is evidenced by the report of Kaur et al. (2008).
Interestingly, the present study concluded that the levels of DA, GSH and 5-HT were comparatively increased and the level was depending upon the concentration of the cardamom supplementation. Whereas, the report of Freitas et al., 2004, Nascimento et al., 2005 clearly indicated that the DA mediated receptor was highly involved for the hyperexcitability of hippocampal and striatal neurons in the brain tissues. Also, the role of serotonergic neurotransmission functions of 5-HT subtype receptors were reported by Petkov et al. (1995). The results of the present study clearly indicated that the treated mice offspring in the water maze study enhanced the number of avoidances in the automatic reflex conditions. These results promisingly concluded that the effect of cardamom on memory enhancement with enhanced serotonergic neurotransmission. Recently, Abu-Taweel et al. (2013) confirmed that the supplementation of carcumin stimulated the learning and memory of mice in the brain hippocampus and this might be because of the antioxidant role of the carcumin and cardamom.
5. Conclusion
The present study concluded that the supplementation of cardamom to the mice have promising activity towards the enhancement of neurotransmitter and also the supplementation had positive effect on enhancing the memory and other behavioral attitudes in the female mice. This positive effect might be the presence of the antioxidant compounds present in the cardamom. Alternatively individual phytochemical constituents present in the cardamom should be evaluated and the neurotransmitter properties of the individual components should be studied in details.
Acknowledgement
The author would like to extend sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no RGP-VPP-240.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelkader S.M., Bauomi A.A., Abdel-Rahman M., Mohammaden T., Rezk M.M. Antioxidant potentials of (Elletaria cardamomum) cardamom against Uranium hazarda. Int. J. Basic Life Sci. 2015;3:64–181. [Google Scholar]
- Aboelnaga S.M. Effect of some levels of cardamom, clove and anise on hepatotoxicity in rats caused by CCL4. World Appl. Sci. J. 2015;33(6):854–865. [Google Scholar]
- Abu-Taweel G.M. Effects of perinatal exposure of lithium on neuro-behaviour of developing mice offspring. Indian J. Exp. Biol. 2012;50(10):696–701. [PubMed] [Google Scholar]
- Abu-Taweel G. Effects of perinatal exposure to Zamzam water in the teratological studies of the mice offspring. Saudi J. Biol. Sci. 2017;14:892–900. doi: 10.1016/j.sjbs.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Taweel G.M., Ajarem J.S., Ahmad M. Neurobehavioral toxic effects of perinatal oral exposure to aluminium on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol. Biochem. Behav. 2012;101(1):49–56. doi: 10.1016/j.pbb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Abu-Taweel G., Ajarem J., Ahmad M. Protective effect of curcumin on anxiety, learning behavior, neuromuscular activities, brain neurotransmitters and oxidative stress enzymes in cadmium intoxicated Mice. J. Behav. Brain Sci. 2013;3(1):74–84. [Google Scholar]
- Ajarem J.S., Ahmad M. Effect of perinatal exposure of Cardamom (Elettaria cadrdamomum) on the post-natal development and social behaviour of mice offspring. J. King Saud Univ. Sci. 1991;4(2):34–57. [Google Scholar]
- Angelo A. Is There an answer? How can a chemically well established antioxidant work differently when in the body? IUBMB Life. 2009;61(12):1159–1160. doi: 10.1002/iub.251. [DOI] [PubMed] [Google Scholar]
- Asha Devi S., Umasankar M.E., Babu S. A Comparative study of antioxidant properties in common Indian spices. Int. Res. J. Pharm. 2012;3(5):465–468. [Google Scholar]
- Campana F., Zervoudis S., Perdereau B., Gez E., Fourquet A., Badiu C., Tsakiris G., Koulaloglou S. Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis. J. Cell. Molec. Med. 2004;8(1):109–116. doi: 10.1111/j.1582-4934.2004.tb00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A.M., Gonçalves L.M., Valente I.M., Rodrigues J.A., Barros A.A. Analysis of cardamonin by square wave voltammetry. Phytochem. Anal. 2011 doi: 10.1002/pca.1370. [DOI] [PubMed] [Google Scholar]
- Coyle I., Wayner M.J., Singer C. Behavioral teratogensis: a critical evaluation. Pharmacol. Biochem. Behav. 1976;4:191–200. doi: 10.1016/0091-3057(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Das I., Acharya A., Berry D.L., Sen S., Williams E., Permaul E., Sengupta A., Bhattacharya S. Antioxidative effects of the spice cardamom against non-melanoma skin cancer by modulating nuclear factor erythroid-2-related factor 2 and NF-κB signalling pathways. Br. J. Nutr. 2012;108(6):984–997. doi: 10.1017/S0007114511006283. [DOI] [PubMed] [Google Scholar]
- Dimitrios B. Sources of natural phenolic antioxidants. Review. Trends Food Sci. Technol. 2006;17:505–512. [Google Scholar]
- Freitas R.M., Vasconcelos S.M.M., Souza F.C.F., Viana G.S.B., Fonteles M.M.F. Monoamine levels after pilocarpine-induced status epilepticus in hippocampus and frontal cortex of Wistar rats. Neurosci. Lett. 2004;370:196–200. doi: 10.1016/j.neulet.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Garg G., Sharma S., Dua A., Mahajan R. Antibacterial potential of polyphenol rich methanol extract of Cardamom (Amomum subulatum) J. Innovative Biol. 2016;3(1):271–275. [Google Scholar]
- Ghosh R., Das A. Synthesis and biological activities of chalcones and their heterocyclic derivatives: a review. World J. Pharm. Pharm. Sci. 2014;3(3):578–595. [Google Scholar]
- Goyal, S.N., Sharma, C., Mahajan, U.B., Patil, C.R., Agrawal, Y.O., Kumari, S., Arya, D.S., Ojha, S., 2015. Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. Int. J. Mol. Sci. 16, 27457–27469. [DOI] [PMC free article] [PubMed]
- Hamzaa R.G., Osman N.N. Using of coffee and cardamom mixture to ameliorate oxidative stress induced in γ-irradiated rats. Biochem. Anal. Biochem. 2012;1:113. [Google Scholar]
- Jeltsch H., Bertrand F., Lazarus C., Cassel J.C. Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiol. Learn. Memory. 2001;76:81–105. doi: 10.1006/nlme.2000.3986. [DOI] [PubMed] [Google Scholar]
- Kaur T., Pathak C.M., Pandhi P., Khanduja K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008;67:25–30. doi: 10.1016/j.bandc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Khan A., Khan Q., Gilani A. Pharmacological basis for the medicinal use of cardamom in asthma. Bangladesh J. Pharmacol. 2011;6:34–37. [Google Scholar]
- Mahindru S.N. Sultanchand & Sons; New Delhi: 1982. Spices in Indian Life. [Google Scholar]
- Mangino M.J., Murphy M.K., Grabau G.G., Anderson C.B. Protective effects of glycerin during hypothermia renal ischemia-reperfusion injury. Am. J. Physiol.—Renal Fluid Elect. Physiol. 1991;261(5):F841–F848. doi: 10.1152/ajprenal.1991.261.5.F841. [DOI] [PubMed] [Google Scholar]
- Nascimento V.S., Oliveira A.A., Freitas R.M., Sousa F.C., Vasconcelos S.M.M., Viana G.S.B. Pilocarpine-induced status epilepticus: monoamine level, muscarinic and dopaminergic receptors alterations in striatum of young rats. Neurosci. Lett. 2005;383:165–170. doi: 10.1016/j.neulet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Nirmala M.A. Studies on the volatile of cardamom (Elleteria cardamomum) J. Food Sci. Technol. 2000;37:406–408. [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ordy J.M., Samorajski J., Collins R.L., Rolstenm G. Pre-natal chlorpromazine wffecte on liver. Survival and behavior of mice offspring. J. Pharmacol. Exptl. Ther. 1966;151:110–125. [PubMed] [Google Scholar]
- Patrick O.E., Hirohisa M., Masahira K., Koreaki M. Central nervous system bioaminergic responses to mechanic trauma. Surg. Neurol. 1991;35:273–279. doi: 10.1016/0090-3019(91)90004-s. [DOI] [PubMed] [Google Scholar]
- Petkov V.D., Belcheva S., Konstantinova E., Kehayov R. Participation of different 5-HT receptors in the memory process in rats and its modulation by the serotonin depleter p-chlorophenylalanine. Acta Neurobiol. Exp. (Wars) 1995;55:243–252. doi: 10.55782/ane-1995-1083. [DOI] [PubMed] [Google Scholar]
- Przygodzka M., Zielinska D., Ciesarová Z., Kukurová K., Zielinski H. Comparison of methods for evaluation of the antioxidant capacity and phenolic compounds in common spices. LWT – Food Sci. Technol. 2014;58:321–326. [Google Scholar]
- Rutten A., Van Albada M., Silveira D.C. Memory impairment following status epilepticus in immature rats: time-course and environmental effects. Eur. J. Neurosci. 2002;16(3):501–513. doi: 10.1046/j.1460-9568.2002.02103.x. [DOI] [PubMed] [Google Scholar]
- Sarkar P.R. V Ed. AMPS Publication, Purulia; West Bengal: 2011. Yaogic Treatment and Natural Remedies; p. 105. [Google Scholar]
- Sengottuvlu, S., 2011. Cardamom (Elettaria cardamomum Linn. Maton) Seeds in Health. Nuts & Seeds in Health and Disease Prevention. Elsevier Inc., pp. 285–291.
- Setty P.V., Sailesh K.S., Neethu, Mukkadan J.K. A study on effect of oral administration of cardamom on memory boosting and regaining in wistar albino rats. Asian J. Health Sci. 2013;1(1):17–20. [Google Scholar]
- Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects. A review. J. Funct. Foods. 2015;18:820–897. [Google Scholar]
- Spiers H.J., Burgess N., Hartley T., Vargha-Khadem F., O’Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11(6):715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- Tariq M., Ahmad M., Moutaery K.A., Deeb S.A. Pentoxifylline ameliorates lithium-pilocarpine induced status epilepticus in young rats. Epilepsy Behav. 2008;12(3):354–365. doi: 10.1016/j.yebeh.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Tavares E.M., Carvalho A.M., Goncalves L.M., Valente I.M., Moreira M.M., Guido L.F., Rodrigues J.A., Doneux T., Barros A.A. Chemical sensing of chalcones by voltammetry: trans-Chalcone, cardamonin and xanthohumol. Electrochim. Acta. 2013;90:440–444. [Google Scholar]
- Vaidya A., Rathod M. An in vitro study of the immunomodulatory effects of Piper nigrum (black pepper) and Elettaria cardamomum (cardamom) extracts using a murine macrophage cell line. Am. Int. J. Res. Formal, Appl. Nat. Sci. 2014;8(1):18–27. [Google Scholar]