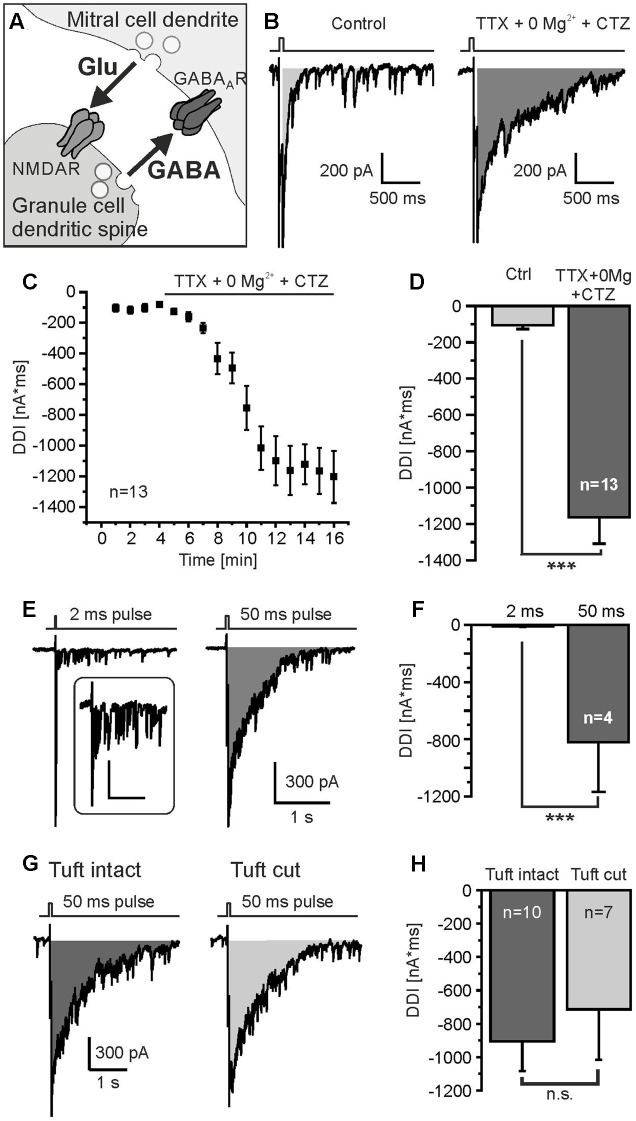
FIGURE 1.
Protocol to measure DDI. (A) Granule cell-mediated DDI is triggered by glutamate release from mitral cell lateral dendrites and subsequent GABA release from granule cell spines. (B) Mitral cells were depolarized from -70 mV to 0 mV for 50 ms, as indicated by the trace on top of the current recording. Tetrodotoxin (TTX, 1 μM) was added to suppress sodium currents, Mg2+ was omitted (0 Mg2+) to increase NMDA receptor-dependent activation of granule cells, and cyclothiazide (CTZ, 200 μM) was added to increase AMPA receptor-dependent activation of interneurons. Under these conditions, DDI was strongly enhanced (right trace). DDI was assessed by calculation of the integral of the current trace within a time window of 2 s after the depolarizing pulse (gray area). (C) Time course of increase in DDI upon wash in of TTX, 0 Mg2+ and CTZ. (D) TTX, 0 Mg2+ and CTZ significantly increased DDI. (E) DDI evoked by a 2-ms depolarization (left trace) and 50-ms depolarization (right trace). Inset: Response to 2-ms depolarization at higher magnification; scale bars: 300 ms, 100 pA. (F) The current integral evoked by a 50-ms depolarization was significantly larger compared to a 2-ms depolarization. (G) DDI in a mitral cell with intact apical dendrite (left trace) and a mitral cell in which the apical tuft was cut during the slicing procedure (right trace). (H) DDI in intact mitral cells was not significantly different compared to mitral cells with cut apical tuft. ∗∗∗p < 0.001; n.s., not significant.