Introduction
Key Teaching Points.
-
•
The present report describes the clinical and electrophysiological features of a rare tachycardia, respiratory cycle–dependent atrial tachycardia (RCAT), from our experience of 3 cases.
-
•
In all cases, RCAT was induced easily under sedation, although atrial pacing with/without isoproterenol or atropine while awake did not induce RCAT sufficiently. In the ablation of RCAT, sedation should be performed if it could not be induced by conventional methods.
-
•
RCAT was successfully eliminated on the earliest activation site by radiofrequency catheter ablation in the 3 cases.
-
•
RCAT onset followed the start of respiration in 2 cases and preceded respiration in 1 case. RCAT probably involves multiple underlying pathophysiological factors.
Focal atrial tachycardia (AT) accounts for 5%–15% of arrhythmias in adults who undergo electrophysiological study (EPS) for paroxysmal supraventricular tachycardia, and it can be generated by enhanced automaticity, triggered activity, and microreentry.1, 2 Radiofrequency catheter ablation (RFCA) has been reported as a curative therapy for AT with a high success rate.1 While almost all instances of AT can be induced by atrial programmed or constant pacing with or without isoproterenol infusion, some ATs with distinct inducibility features have been reported. Swallowing-induced AT is one of the most well-known ATs of unusual presentation.3, 4 Activation mapping of swallowing-induced AT requires patients to swallow during mapping because it typically cannot be induced by any pacing methods or isoproterenol, and can only be induced by deglutition. RFCA is also effective in eliminating swallowing-induced AT, similar to typical AT.3, 4 Therefore, understanding how to induce targeted AT is important to the success of RFCA.
Another atypical form of AT, respiratory cycle–dependent AT (RCAT), is a rare clinical condition, the mechanism of which has not been clarified. This report describes successful induction and elimination of RCAT in 3 patients in whom different mechanisms underlying the appearance of RCAT were confirmed.
Case report
Methods of observation, EPS, and RFCA
Three patients were diagnosed with RCAT and treated with EPS and RFCA. We defined RCAT as AT or short run with P wave differing from sinus rhythm that repetitively appears linked with inspiration (Figure 1).
Figure 1.
Representative electrocardiograms of respiratory cycle–dependent atrial tachycardia (RCAT). A 12-lead electrocardiogram shows incessant atrial short runs linked with respiration induced after sedation. CVP = central venous pressure.
The patients in cases 1 and 2 were prescribed flecainide and bisoprolol before RFCA, which were discontinued for 1 week prior to RFCA. Initially, no sedative or opioid was used, only local anesthesia. The filter setting was 30–150 Hz in cases 1 and 2 and 30–500 Hz in case 3. A duodecapolar catheter was inserted via the right subclavian vein into the coronary sinus, and a quadripolar or decapolar catheter was inserted into the right ventricle, para-Hisian region, and high right atrium via the right femoral vein. Three-dimensional electroanatomic mapping was used in all 3 cases. After insertion of the electrode catheters, induction of AT was attempted by atrial programmed or constant pacing. If AT was not induced, 3–5 μG/min of isoproterenol was infused, and induction by pacing was likewise performed. If initial induction failed, patients were given propofol and pentazocine infusion. After induction of RCAT, activation mapping was performed, and RFCA was applied to the earliest site. The application cycle of RFCA was 30 seconds with a power setting of 25–30 W, and additional RF applications were performed if the application seemed to be effective. After the RFCA sessions, all patients were followed up with repeat 12-lead electrocardiogram at each hospital visit. No patients were prescribed antiarrhythmic drugs after the procedure. All patients underwent 24-hour Holter monitoring after the procedure. All patients gave written informed consent to participate in this observation, and the ethics committees of Saitama Red Cross Hospital and Musashino Red Cross Hospital approved this study.
Presentation of the representative case: Case 1
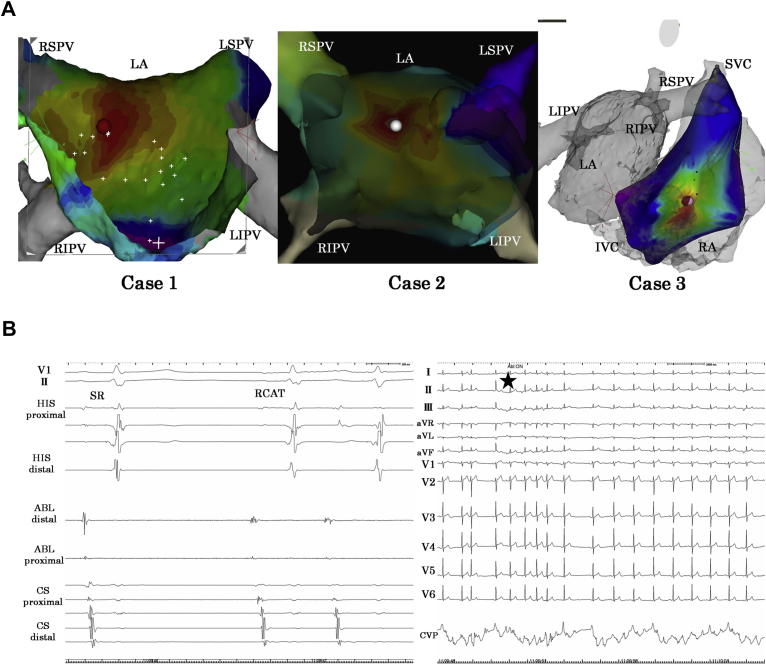
A 46-year-old man was referred for treatment of palpitations lasting for several seconds, which occurred during deep inspiration or vocalizing. Twenty-four-hour Holter monitoring revealed repetitive 5- to 10-beat short atrial runs with his symptoms. Beta blocker and sodium channel blocker were not effective for his symptoms. He underwent EPS and RFCA. No atrial firing was induced by a high dose of isoproterenol or atropine infusion and atrial burst stimulation. After induction of anesthesia by propofol with pentamidine infusion, he began to snore upon depression of the tongue, and clinical AT repeatedly appeared linked to breathing (Figure 1). Only a low dose of oxygen was given via a nose cannula because of a slight decrease in oxygen saturation. The onset of AT occurred after the initiation of thoracic negative pressure resulting from airway narrowing upon depression of the tongue. Activation mapping showed the earliest site of activation localized to the posterior wall of the left atrium (Figure 2A, left). A single application of radiofrequency energy at 30 W was performed at this site, without change of sinus cycle length or atrioventricular conductivity during ablation, and RCAT was immediately eliminated. Since this session, the patient has remained free from palpitations over a 24-month follow-up period.
Figure 2.
Three-dimensional electroanatomic mappings and local intracardiac electrocardiograms of the cases. A: Three-dimensional electroanatomic mapping obtained in the anterior-posterior view showed the earliest activation sites of catheter ablation in case 1 (left) and case 2 (center). In case 3 (right), the earliest activation site was located in the low lateral right atrium (RA). In all 3 cases, radiofrequency ablation of the sites eliminated respiratory cycle–dependent atrial tachycardia (RCAT). IVC = inferior vena cava; LA = left atrium; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava. B: Electrocardiogram shows that fragmented potential was detected at the earliest activation site (left); RCAT was eliminated during radiofrequency ablation of the site (right). ABL = ablation catheter; CS = coronary sinus; HIS = His bundle.
Summary of the cases
The results of the 3 cases are summarized in Table 1. All 3 patients were male, and 2 of them had ineffective medical treatment with beta blockers and sodium channel blockers. The cycle lengths of the ATs were 285–340 msec. Notably, in no case was RCAT induced sufficiently by atrial pacing, isoproterenol, or atropine. RCAT spontaneously occurred only under sedation. After the appearance of RCAT, neither isoproterenol nor atropine appeared to affect it. In 2 cases RCAT appeared after the onset of respiration, and in the remaining case it appeared before respiration onset (Figure 3). Mechanical movement on positive-pressure ventilation could not induce RCAT in 2 cases, and positive-pressure ventilation was not performed in the final case. The earliest activation site of RCAT was the posterior left atrium in 2 cases and low lateral right atrium in 1 case, with centrifugal activation patterns in all the cases (Figure 2A). The earliest sites activated 14–28 msec earlier than coronary sinus ostium. All RCATs were completely eliminated after RFCA to the earliest activation sites (Figure 2B). In all the cases, RFCA of the earliest activation site did not induce any change of sinus rate or atrioventricular conductivity. No recurrences were found in any of the patients during follow-up periods. Follow-up periods were 24 months for case 1, 20 months for case 2, and 12 months for case 3. All cases were followed by 24-hour Holter monitoring and all 3 patients have remained free from AT recurrence.
Table 1.
Summary of the 3 cases
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Onset age/sex | 44/male | 70/male | 55/male |
| Previous failed medication | Beta blocker Na channel blocker |
Beta blocker Na channel blocker |
Not performed |
| P-wave morphology of V1 | + | + | +/− |
| State of occurrence | Only under sedation | More under sedation | Only under sedation |
| Inducibility of atrial pacing | Not induced | Not induced | Not induced |
| Inducibility/response to ISP | Not induced/not changed | With pacing/not changed | Not induced/not performed |
| Inducibility/response to atropine | Not induced/not changed | Not induced/not changed | Not induced/not performed |
| Onset of AT | Behind respiration | Ahead of respiration | Behind respiration |
| Inducibility of positive-pressure ventilation | Not performed | Not induced | Not induced |
| Earliest activation site | Posterior LA | Posterior LA | Low RA |
AT = atrial tachycardia; ISP = isoproterenol; LA, left atrium; Na = sodium; RA = right atrium.
Figure 3.
Relationship between tachycardia onset and respiration. Electrocardiograms of tachycardia initiation and central venous pressure (CVP) in 2 cases, illustrating preceding sinus beats followed by respiratory cycle–dependent atrial tachycardia (RCAT). In case 1, the initiation of RCAT (dotted line) was followed by depression of CVP (arrow); contrarily, in case 2, the initiation of RCAT occurred prior to depression of CVP.
Discussion
The first case of RCAT was described by Takatsuki and colleagues5 in 2001, coexisting with Wolff-Parkinson-White syndrome and treated by RFCA, and there have been several subsequent reports of RCAT.6, 7 Yamamoto and colleagues8 demonstrated precisely the clinical characteristics, geometry of origin, EPS, and outcome of RFCA in 7 cases with 9 RCATs. They reported that RCAT appeared to arise from the right pulmonary vein and superior vena cava, and suggested that the mechanism of RCAT was triggered by autonomic nervous system activity modulated by respiration or chest movement itself. In 1 of our cases, RCAT originated from the right pulmonary venous antrum, near the area described in this previous study,8 but the earliest sites of the other 2 cases were localized to the left posterior atrium and low lateral right atrium. Thus, RCAT can appear from a wider area of the atrium than was previously thought. Furthermore, in cases 2 and 3, positive-pressure ventilation was performed, but RCATs were not induced. This result indicates that some RCATs are not induced by chest respiratory movement, but only by spontaneous respiration.
The present study is the first report showing the effect of sedation for induction of RCAT. While patients were awake, RCATs were not induced by atrial pacing with isoproterenol, although RCATs spontaneously appeared during sedation in all 3 cases. These results might be related to the fact that emotional tension strongly suppressed RCATs, and the underlying mechanism of RCAT could be related to sympathetic and parasympathetic activation.
The other new insight regarding RCAT in this study is that RCAT onset can vary in reference to the beginning of respiration. In cases 1 and 3, the onset of all RCATs followed the previous inspiration and decreasing thoracic pressure. The mechanism of RCAT was understood as an autonomic nervous reflex stimulated by inspiration or mechanical stimulation by the breathing action. However, interestingly, the onset of all RCATs in case 2 was preceded by the beginning of respiration. The finding that RCAT occurred before inspiration indicates a different etiology from cases 1 and 3, and the difference between the 2 patterns indicates that multiple mechanisms of RCAT are likely to exist. We hypothesize that the cause underlying the earlier RCAT occurrence in case 2 was an autonomic nervous reflex using another neural connection or that efferent fibers of respiratory muscles such as the phrenic nerve directly connected to cardiovascular fibers. Another factor possibly related to RCAT is the atrial neural network and ganglionated plexi. Previous studies have reported that stimulation of the atrial neural network can affect sinus node rate and atrioventricular conduction.9, 10 In these cases, RFCA of RCAT foci did not induce any change of sinus rate or atrioventricular conductivity. The cases described here suggest that RCAT probably involves multiple underlying pathophysiological factors, but the mechanisms of this arrhythmia remain largely unclear. Further exploration of the cardiovascular, respiratory, and automatic nervous systems is needed to clarify these mechanisms.
References
- 1.Chen S.A., Chiang C.E., Yang C.J., Cheng C.C., Wu T.J., Wang S.P., Chiang B.N., Chang M.S. Sustained atrial tachycardia in adult patients. Electrophysiological characteristics, pharmacological response, possible mechanisms, and effects of radiofrequency ablation. Circulation. 1994;90:1262–1278. doi: 10.1161/01.cir.90.3.1262. [DOI] [PubMed] [Google Scholar]
- 2.Roberts-Thomson K.C., Kistler P.M., Kalman J.M. Focal atrial tachycardia I: clinical features, diagnosis, mechanisms, and anatomic location. Pacing Clin Electrophysiol. 2006;29:643. doi: 10.1111/j.1540-8159.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi Y., Aonuma K., Sekiguchi Y., Higuchi K., Obayashi T., Isobe M. Curative therapy for swallowing-induced tachycardia by pulmonary vein antrum isolation. J Cardiovasc Electrophysiol. 2005;16:1370–1374. doi: 10.1111/j.1540-8167.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 4.Tada H., Kaseno K., Kubota S., Naito S., Yokokawa M., Hiramatsu S., Goto K., Nogami A., Oshima S., Taniguchi K. Swallowing-induced atrial tachyarrhythmias: prevalence, characteristics, and the results of the radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2007;30:1224–1232. doi: 10.1111/j.1540-8159.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- 5.Takatsuki S., Mitamura H., Miyoshi S., Ogawa S. Respiratory cycle–dependent left atrial tachycardia. J Cardiovasc Electrophysiol. 2001;12:1202. doi: 10.1046/j.1540-8167.2001.01202.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin P.H., Huang J.L., Ting C.T., Chen S.A. Respiration and initiation of atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:979. doi: 10.1046/j.1540-8167.2001.00979.x. [DOI] [PubMed] [Google Scholar]
- 7.Müssigbrodt A., Hindricks G., Bollmann A. Respiratory cycle-dependent left atrial tachycardia in a former Tour de France cyclist. Europace. 2015;17:387. doi: 10.1093/europace/euu399. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T., Hayashi M., Miyauchi Y., Murata H., Horie T., Igawa O., Kato T., Mizuno K. Respiratory cycle–dependent atrial tachycardia: prevalence, electrocardiographic and electrophysiologic characteristics, and outcome after catheter ablation. Heart Rhythm. 2011;8:1615–1621. doi: 10.1016/j.hrthm.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa H., Scherlag B.J., Patterson E., Ikeda A., Lockwood D., Jackman W.M. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6:S26–34. doi: 10.1016/j.hrthm.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Hou Y., Scherlag B.J., Lin J., Zhou J., Song J., Zhang Y., Patterson E., Lazzara R., Jackman W.M., Po S.S. Interactive atrial neural network: determining the connections between ganglionated plexi. Heart Rhythm. 2007;4:56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]