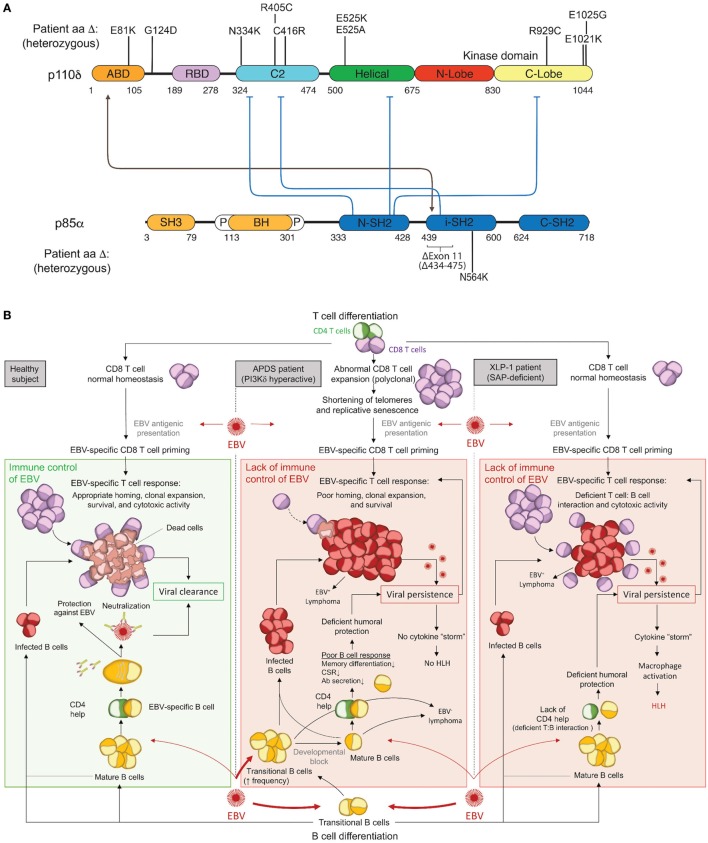
Figure 1.
Activated PI3Kδ Syndrome (APDS) GoF mutations in the PI3Kδ complex and associated immune dysfunction responsible for Epstein–Barr virus (EBV) susceptibility. (A) Schematic representation of p110δ and p85α protein domains and APDS mutations reported in patients. The black line depicts the stabilizing interaction, and the blue lines show the inhibitory contacts within the PI3Kδ complex. ABD, adaptor-binding domain; BH, breakpoint-cluster region homology domain; P, proline-rich region; SH, SRC-homology domain; N, amino-terminal; i, inter; C, carboxy-terminal. (B) Schematic representation of the current understanding for the immune control of EBV in healthy subjects (left) and proposed hypothesis for EBV susceptibility in APDS (middle) and XLP1 (right) patients. APDS mutations cause abnormal polyclonal expansion of CD8 T cells that become senescent. Senescent CD8 T cells show an impaired EBV-specific response due to limited homing, expansion, and survival. In conjunction with CD8 T-cell defects, APDS patients exhibit an elevated frequency of transitional B cells, a major cell type for cell entry of EBV, and have defective humoral immunity that may further contribute to EBV susceptibility. In comparison, XLP1 patients, who are susceptible to EBV and develop HLH, are deficient in the SAP adaptor and exhibit defective EBV-specific T cell: B-cell interactions, causing a lack of CD4 help and a failure of CD8 T-cell cytotoxicity. As opposed to APDS, viral persistence in XLP1 patients causes a recurring stimulation/expansion of EBV-specific CD8 T cells and results in a cytokine storm underlying hemophagocytic lymphohistiocytosis (HLH). Antibodies depiction: taken from SMART (Servier Medical Art) licensed under a Creative Commons Attribution 3.0 Unported License.