Figure 1.
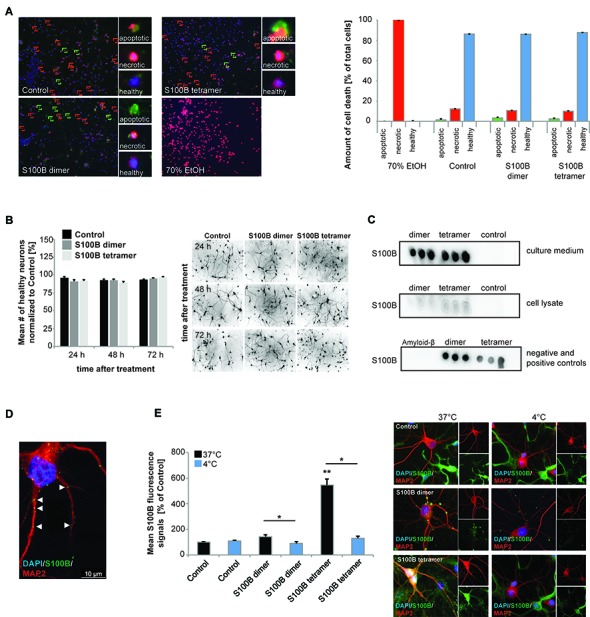
Exogenous application of S100B is non-toxic and leads to active uptake of the protein into cells. Treatment of hippocampal cultures with 30 μM dimeric and with 30 μM tetrameric S100B for 24 h at DIV10 was performed. (A) No significant differences in cell health were detected after application of 30 μM S100B dimer and tetramer. Apoptotic cells were identified using Annexin V—FITC (green signals), necrotic cells (red signals) by ethidium homodimer III and the total number of cells (blue signals) was assessed using Hoechst 33342 labeling all nuclei. A total of six optic fields of view per condition were analyzed. Ethanol treatment was used as positive control (Welch’s ANOVA, F = 312.579; p < 0.001; Post hoc analysis: control healthy cells vs. dimer healthy cells, p = 1.000; control healthy cells vs. tetramer healthy cells, p = 0.965; control healthy cells, dimer healthy cells, and tetramer healthy cells vs. 70% EtOH healthy cells, p < 0.001). (B) One time treatment of hippocampal neurons for 24 h, 48 h and 72 h also reveals no significant influence of S100B on cell health (one way ANOVA, F = 1.824; p = 0.085), assessed by DAPI staining of nuclei and visualization of dendrites by MAP2. Right panel: exemplary images showing MAP2 staining. (C) Dot-blot experiments show that after application of exogenous S100B dimer and tetramer, a high amount of proteins remains extracellular (upper panel). Weaker signals observed when cell lysates are used indicate that some of the protein is taken up into cells (middle panel). Medium and lysate of untreated cells were used as control. To confirm specificity of the antibody reaction, purified Amyloid-β, S100B dimer and tetramer were used (lower panel). (D,E) Hippocampal neurons show S100B immunoreactive puncta within their dendrites and cell soma. (D) Confocal fluorescent microscopy using z-stacks confirms the presence of intracellular exogenously applied S100B protein in vesicular like structures. (E) The S100B signal of neuronal soma increases significantly after application of exogenous S100B. This increase is abolished by interference with endocytotic processes by temperature reduction (Welch’s ANOVA, F = 31.823; p < 0.001; Post hoc analysis: control 37°C vs. S100B dimer 37°C, p = 0.061; S100B dimer 37°C vs. S100B dimer 4°C, p = 0.021; control 37°C vs. S100B tetramer 37°C, p = 0.004; S100B tetramer 37°C vs. S100B tetramer 4°C, p = 0.043). For quantification 10 cells per optic field and 5 optic fields per conditions were analyzed. Exemplary images are shown in the right panel. Neurons were visualzed using anti-MAP2 staining (red). Cell nuclei were labeled with DAPI (blue), and S100B (green) was labeled using anti-S100B antibody. MAP2 negative and S100B positive cells are astrocytes present in the cultures.
