Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare hyperinflammatory syndrome characterized by fever, pancytopenia and splenomegaly. The underlying hemophagocytosis occurs primarily in the bone marrow, liver and lymph nodes. Multiple microbiological agents, including cytomegalovirus, Epstein-Barr virus and Mycobacterium tuberculosis, have been implicated in the pathogenesis of HLH. The present study presents a case of HLH associated with Leuconostoc pseudomesenteroides infection treated successfully with clindamycin. A 33-year-old man presented with recurrent episodes of fever and diarrhea. Upon initial treatment at another hospital (the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China), blood chemistry analysis demonstrated moderate anemia (hemoglobin 88 g/l; reference range, 120.0–160.0), elevated ferritin (1,068.47 mg/l; reference range, 21.81–274.66), total bilirubin (392.4 mmol/l; reference range, 5.1–28.0), conjugated bilirubin (335.7 mmol/l; reference range, 0–10.0), and γ-glutamyl transpeptidase (150 U/l; reference range, 10–60). The patient was treated with antibiotics for suspected pneumonia and cholecystitis, but new symptoms (including diarrhea and inflammatory colitis) started to emerge. The patient was subsequently treated with ganciclovir (5 mg/kg/day for 1 month), but body temperature increased to 41.0°C. Upon transferring to our hospital, the patient had severe anemia (hemoglobin, 39 g/l; red blood cell, 1.61×1012/l; reference range, 4.0–5.5×1012/l). Jaundice was apparent: Total bilirubin, 299.5 mmol/l; conjugated bilirubin, 215.7 mmol/l. The patient was treated with clindamycin (150 mg, taken orally every 12 h for 1 week) and supportive care that included parenteral nutrition. Symptoms rapidly dissipated after the treatment. Blood chemistry analysis 5 days after the first dose of clindamycin revealed substantial improvement in anemia and jaundice. The patient requested discharge for financial reasons, but continued treatment (details not available) at a local hospital (Pengpai Memorial Hospital, Shanwei, China). Upon a visit to our hospital 8 months later, the patient has no notable complaints, with the exception of moderate anemia. The present case suggests that HLH may be associated with L. pseudomesenteroides infection.
Keywords: hemophagocytic lymphohistiocytosis, Leuconostoc pseudomesenteroides, hyperbilirubinemia, hepatomegaly, splenomegaly, clindamycin
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare hyperinflammatory syndrome that is typically characterized by fever, pancytopenia, and splenomegaly (1). Other features include hypertriglyceridemia, hypofibrinogenemia, liver dysfunction, and elevated ferritin (1). A major underlying pathology is hemophagocytosis, which mainly occurs in the bone marrow, spleen and lymph nodes (1). Hemophagocytic syndrome may be associated with malignancies (malignancy-associated hemophagocytic), hereditary diseases and autoimmune diseases (2). It is also linked with Epstein-Barr virus (EBV) infection (2), parainfluenza virus infection (3), cytomegalovirus (CMV) infection (4) and bacterial infection caused by Mycobacterium tuberculosis (M. tuberculosis) (5) and Mycoplasma pneumonia (6). Hemophagocytic syndrome that is associated with infections has been suggested to be a result of immunological activation of the immune system, and is sometimes referred to as secondary HLH (sHLH) (1). Pathologically, hemophagocytosis occurs in the bone marrow and other tissues, where activated macrophages engulf erythrocytes, leukocytes, platelets and their precursors (2). An improved understanding of the pathophysiology of HLH may aid to clarify the interactions between the immune system and infectious agents (2).
Leuconostoc pseudomesenteroides infection typically occurs in patients with underlying diseases or a history of vancomycin use (7–9). It was first reported in humans in 2 immuno-compromised patients in 1985 (7), and later observed in patients receiving solid organ transplants (8). An outbreak of Leuconostoc mesenteroides (a subspecies of Leuconostoc) involving 6 patients receiving parenteral nutrition in northwest Spain has also been reported (9). However, there has been no report of acquired HLH secondary to Leuconostoc pseudomesenteroides infection to the best of our knowledge.
In the present report, we present a case of sHLH with L. pseudomesenteroides bacteremia. The patient presented with recurrent episodes of fever, diarrhea, severe anemia, liver dysfunction and elevated levels of ferritin. HLH associated with non-viral pathogens often respond to treatment of the underlying infection (2). The present case was successfully managed with clindamycin. The present findings expand the spectrum of possible microbiological agents implicated in hemophagocytic syndrome.
Case report
A 33-year old man presented with recurrent episodes of fever and diarrhea for 3 months. The patient was treated at, and discharged from another hospital (the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China) before presenting to us (the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China). Previous medical history included unexplained anemia and splenomegaly. The patient denied previous blood transfusion. The patient was married and had 2 children and there was no family history of familial disease except for thalassemia in a sister. The patient provided written informed consent for the presentation of his case as a report.
The fever (>40°C) started 3 months prior to admission to our hospital, and the patient had received medical attention at the First Affiliated Hospital of Sun Yat-sen University 7 days after the first presentation of symptoms. A blood chemistry panel conducted at the First Affiliated Hospital of Sun Yat-sen University included hemoglobin (88 g/l reference range, 120.0–160.0), ferritin (1,068.47 mg/l; reference range, 21.81–274.66), total bilirubin (392.4 mmol/l; reference range, 5.1–28.0), conjugated bilirubin (335.7 mmol/l; reference range, 0–10.0), γ-glutamyl transpeptidase (γ-GT; 150 U/l; reference range, 10–60) and fibrinogen (1.53 g/l; reference range, 2.0–4.0). The medical record revealed that the patient received mechanical ventilation from day 1 to day 16 after admission for respiratory failure. The patient received antibiotic treatment for suspected pneumonia and cholecystitis. After 10 days, the patient developed diarrhea with watery stool (4–6 l/day) but no mucus or blood. He received treatment with itraconazole and norvancomycin, (details not available) but did not improve. Colonoscopy 6 days later identified inflammatory colitis. Immunohistochemical analysis of colon biopsy specimen identified CMV. Treatment with ganciclovir was initiated at a dose of 5 mg/kg/day for 1 month and the patient markedly improved. Abdominal pain and bloating dissipated, and body temperature returned to normal. However, diarrhea did not improve. Immunohistochemical examination (details not available) of the peripheral blood 2 weeks after the initiation of ganciclovir treatment revealed no CMV. Bone marrow aspiration (details not available) identified no hemophagocytosis. Blood chemistry revealed improvement in total bilirubin (86.7 mmol/l), conjugated bilirubin (43.9 mmol/l), γ-GT (71 U/l) and fibrinogen (1.24 g/l). The patient requested discharge from the hospital.
On day 3 after discharge from the First Affiliated Hospital of Sun Yat-sen University, the patient presented to us at the First Affiliated Hospital of Guangzhou University of Chinese Medicine. His body temperature was 41.0°C. Physical examination identified jaundice of the skin and sclera. The lower abdomen was distended, but no tenderness or rebound tenderness was evident. Physical examination determined that hepatomegaly and splenomegaly were evident. A bedside chest X-ray identified no abnormality. Nutritional support (liquid supplements since day 1 after admission) was provided via a gastric tube. Blood chemistry analysis identified anemia (hemoglobin, 36 g/l) and hyperbilirubinemia (total bilirubin, 234.9 mmol/l; conjugated bilirubin, 147.5 mmol/l). The fever persisted (40°C) and the patient was transferred to the intensive care unit 10 days after admission. CMV-immunoglobulin M testing (cat. no. 315A; DIESSE Diagnostica Senese S.p.A., Siena, Italy) of the peripheral blood yielded a negative result. T lymphocyte count was measured, including cluster of differentiation (CD)3+ (79.2.2%; reference range, 50–84%), CD4+ (38.2%; reference range, 27–51%), CD8+ (35.3%; reference range, 15–44%), T helper/suppressor cell ratio (1.08; reference range, 0.71–2.78). The patient's hemoglobin was 39 g/l. Red blood cell count was 1.61×1012/l (reference range, 4.0–5.5×1012/l); leukocyte count was 2.67×109/l (reference range, 2.0–7.5×109/l); and platelet count was 57×109/l (reference range, 100–400×109/l). Ferritin was substantially elevated (7,931.21 mg/l). Blood chemistry analysis identified a total bilirubin value of 299.5 mmol/l and conjugated bilirubin value of 215.7 mmol/l. Urinalysis was conducted according to the AVE 764+752 (Fully Automated Urinalysis system; AVE Science & Technology Co., Ltd., Changsha, China); The dipstick test for occult blood detected the peroxidase activity of RBCs. The following scoring system was used: +, indicated >3 RBCs was present and therefore this suggested the urine was positive for blood; ++, indicated high blood content. The normal reference value of urinary occult blood was 0 (−, negative) and the normal reference value of urinary bilirubin was 0 (−, negative). Furthermore, normal reference value of erythrocytes: 0–1 RBCs/high power lens field (HPF).
Urinalysis identified occult blood (++), urinary bilirubin (++) and 10 erythrocytes/HPF. An abdominal computed tomography (CT) scan identified splenomegaly, multiple gall bladder stones and inflammatory changes in the gall bladder (gall bladder was enlarged, the wall was thickened). Wright-Giemsa staining was performed at room temperature for 10 min on bone marrow smears using Wright stain (cat. no. MKBH5619; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Samples were viewed using a light microscope (magnification, ×1,000). A bilateral iliac crest bone marrow biopsy identified active hemophagocytosis and phagocytosis of nucleated red blood cells and platelets (Fig. 1). A whole-body positron emission tomography/CT scan revealed no tumor mass. Peripheral blood and bone marrow aspiration sample specimens were obtained using a strict aseptic technique and injected into culture agar (blood agar, cat. no. 1004144770; bioMerieux, Craponne, France). The positive cells (1.5×106 cells per plate) were cultured in a Colombian blood plate and the bacteria were grown for 48 h. L. pseudomesenteroides was identified using a VITEK 2 GP Test kit (cat. no. 242347540, microbial identification platform, VITEK2 Compact system; BioMerieux). Results from a stool culture for Clostridium difficile (C. difficile) was negative. A blood and bone marrow aspirate culture were obtained on day 10 after admission, and results came back positive on day 14 after admission for L. pseudomesenteroides. The Kirby-Bauer disc method was used to conduct a drug sensitivity test using Mueller-Hinton blood agar plates and disks filled with specific antibiotics, including penicillin (10 units), xefotaxime (30 µg), cefepime (30 µg), clindamycin (2 µg), levofloxacin (5 µg), chloramphenicol (30 µg) vancomycin (30 µg), linezolid (30 µg), ampicillin (10 µg) and ceftriaxone (30 µg). Plates were incubated at 37°C for 24 h. Subsequently, the diameters of the zone of inhibition (ZOI) were calculated. The drug sensitivity test indicated that L. pseudomesenteroides was vancomycin-resistant and clindamycin-sensitive. In addition to clindamycin (150 mg, taken orally every 12 h for 1 week), the patient received 4 units of red blood cell suspension and 350 ml frozen plasma and partial parenteral nutrition. The fever subsided rapidly (37.0°C) after 1 day of clindamycin therapy and the patient became afebrile after 7 days of clindamycin therapy. A repeat blood culture 10 days later identified L. pseudomesenteroides. Blood chemistry 5 days later revealed improvement in hemoglobin (77 g/l), red blood cell count (3.05×1012/l), platelet count (139×109/l), and total (71.5 mmol/l) and conjugated bilirubin (45.2 mmol/l). Body temperature returned to normal (36.5°C) and the volume of stool was reduced to 350 ml/day. A repeat blood culture 2 weeks later was negative for L. pseudomesenteroides. One week later, the patient requested discharge due to financial concern.
Figure 1.
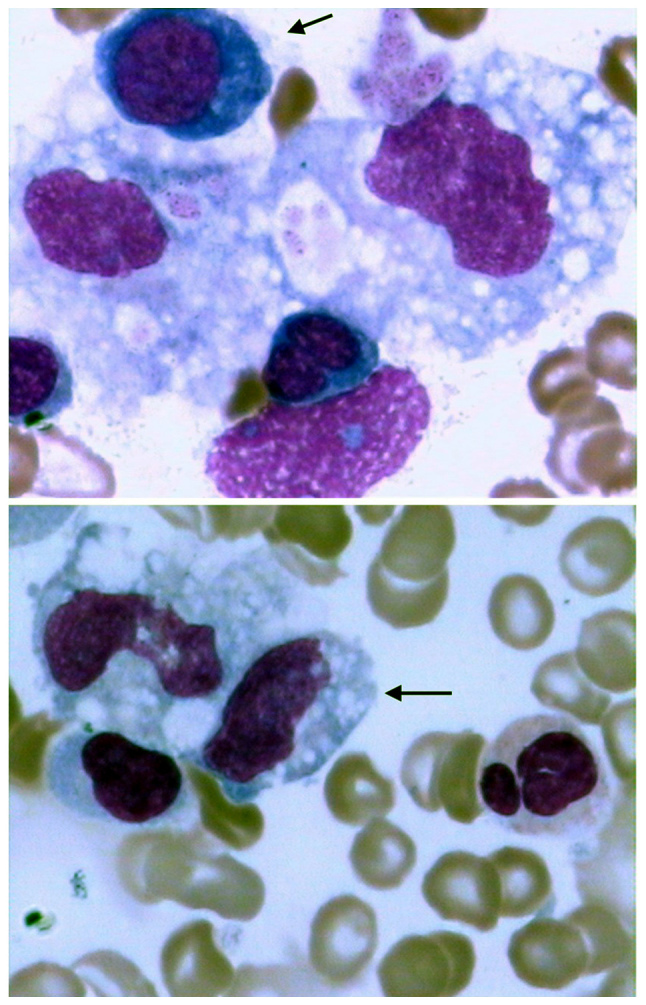
Hemophagocytosis in the bone marrow during Leuconostoc pseudomesenteroides-associated hemophagocytic lymphohistiocytosis. Hypocellularity and active hemophagocytosis (arrow in the upper and lower panel) were identified in bone marrow aspirate. Magnification, ×1,000. Wright-Giemsa stain indicated phagocytosis of nucleated red blood cells and platelets. Cytoplasm of basophilic erythroblasts were indicated blue and nuclei of basophilic erythroblast were indicated purple.
A telephone follow-up 2 weeks later revealed that the patient continued treatment (details not available) at Pengpai Memorial Hospital (Shanwei, China). Blood culture for L. pseudomesenteroides was negative based on patient disclosure. The patient visited our hospital 8 months later and a complete blood count test was performed; he continued to be anemic (hemoglobin at 89 g/l) but had no other complaints.
Discussion
Hemophagocytic lymphohistiocytosis is a hyperinflammatory syndrome that may become fatal if not treated appropriately. Multiple microbiological agents, including CMV, EBV and M. tuberculosis have been implicated in the pathogenesis of HLH (3–6). HLH has predominantly been reported in children (10), but may occur in people of all ages. The current report indicates that L. pseudomesenteroides infection may also be associated with HLH.
To the best of our knowledge, fewer than 10 cases of L. pseudomesenteroides infections have been reported so far (11–14), all of which occurred in adults. Patients who have received vancomycin are particularly vulnerable (7–9). The patient in the present case received multiple antibiotics for suspected pneumonia and cholecystitis, and subsequently norvancomycin for suspected C difficile pseudomembranous colitis. The patient exhibited clinical, laboratory and histopathological abnormalities (Table I) indicative of hemophagocytic lymphohistiocytosis, according to previously described diagnostic guidelines (1).
Table I.
Revised diagnostic guidelines for HLH.
| The diagnosis of HLH may be established if either i) or ii) is fulfilled |
|---|
| i) A molecular diagnosis consistent with HLH |
| Mutations in the gene encoding perforin (PRF) |
| Mutations in the gene UNC13D (17q25) |
| STX11 on chromosome 6q24 |
| ii) Diagnostic criteria for HLH fulfilled (five out of the eight criteria below) |
| Initial diagnostic criteria (to be evaluated in all patients with |
| HLH) |
| Fevera |
| Splenomegalyb |
| Cytopenia (affecting ≥2 of 3 lineages in the peripheral blood) |
| Hemoglobin <90 g/l (in infants <4 weeks: Hemoglobin, <100 g/l)c |
| Platelets, <100×109/ld |
| Neutrophils, <1.0×109/l |
| Hypertriglyceridemia and/or hypofibrinogenemia |
| Fasting triglycerides, ≥3.0 mmol/l (i.e., ≥265 mg/dl) |
| Fibrinogen, ≤1.5 g/le |
| Hemophagocytosis in bone marrow or spleen or lymph nodesf |
| No evidence of malignancy |
| New diagnostic criteria |
| Low or absent NK-cell activity |
| Ferritin, ≥500 mg/lg |
| Soluble CD25 (i.e., soluble IL-2 receptor), ≥2,400 U/ml |
Fever, the maximum body temperature of the patient was 41.0°C.
Splenomegaly, abdominal CT scan identified splenomegaly.
Hemoglobin, the lowest hemoglobin of the patient was 39 g/l.
Platelets, the lowest platelets of the patient was 57×10e9/l.
Fibrinogen, the lowest fibrinogen of the patient was 1.24 g/l.
Hemophagocytosis in bone marrow. The result was shown by bone marrow smears using Wright stain (Fig. 1).
Ferritin, the highest ferritin of the patient was 1,068.47 mg/l. The patient was assessed according to the revised diagnostic guidelines for HLH i). The patient in the present case met criteria a-f above. HLH, hemophagocytic lymphohistiocytosis; NK, natural killer; CD, cluster of differentiation; IL, interleukin.
A number of cases of Leuconostoc spp. infection have previously been reported (15–19). In contrast, few cases of L. pseudomesenteroides infection have been documented. L. pseudomesenteroides infection may occur in patients with underlying diseases and/or a history of vancomycin use (11,12). Cappelli et al (11) reported 5 clinical isolates of L. pseudomesenteroides in nosocomially acquired urinary tract infections; all 5 isolates were sensitive to clindamycin. The patient in the present report had suspected cholecystitis and received norvancomycin. Furthermore, a drug sensitivity test in our hospital indicated resistance to vancomycin. Tholpady et al (12) observed that Leuconostoc sepsis in a 64-year-old liver transplant recipient following extended exposure to vancomycin.
L. pseudomesenteroides infections are typically treated with clindamycin, as in the current case and other reported cases (8,12). The patient in the present case responded well to clindamycin. The fever subsided (37.0°C) after 1 day of clindamycin therapy and the patient became afebrile after 7 days of clindamycin therapy. Although the patient remained positive for L. pseudomesenteroides 10 days later, clinical features (as reflected by liver function and hemoglobin) improved substantially. A blood culture 2 weeks later was negative for L. pseudomesenteroides.
L. pseudomesenteroides is intrinsically resistant to vancomycin and the majority of reports indicate that it is sensitive to clindamycin (8,12). Therefore, appropriate antibiotic therapy based on drug sensitivity results should be administered, and in the absence of drug sensitivity data, clindamycin may be preferred. Supportive therapy and treatment of the underlying diseases should also be initiated.
In conclusion, HLH may be associated with L. pseudomesenteroides infection. The present case expanded the spectrum of microbiological agents implicated in HLH and suggested that such patients may respond to treatment with clindamycin.
References
- 1.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 2.Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. 2000;6:601–608. doi: 10.3201/eid0606.000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beffermann N, Pilcante J, Sarmiento M. Acquired hemophagocytic syndrome related to parainfluenza virus infection: Case report. J Med Case Rep. 2015;9:78. doi: 10.1186/s13256-015-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halfon P, Retornaz F, Mathieu D, Helbert T, Philibert P, Pégliasco H. Virus-associated hemophagocytic syndrome related to acute CMV and HBV sexual co-infection: A case report. J Clin Virol. 2009;46:189–191. doi: 10.1016/j.jcv.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Hui YM, Pillinger T, Luqmani A, Cooper H. Haemophagocytic lymphohistiocytosis associated with Mycobacterium tuberculosis infection. BMJ Case Rep. 2015;2015:bcr2014208220. doi: 10.1136/bcr-2014-208220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibino M, Sato S, Shimizu T, Yamamoto S, Ohe M, Kondo T. Hemophagocytic lymphohistiocytosis secondary to Mycoplasma pneumoniae infection without pneumonia. Intern Med. 2014;53:1679–1683. doi: 10.2169/internalmedicine.53.2089. [DOI] [PubMed] [Google Scholar]
- 7.Buu-Hoï A, Branger C, Acar JF. Vancomycin-resistant streptococci or Leuconostoc spp. Antimicrob Agents Chemother. 1985;28:458–460. doi: 10.1128/AAC.28.6.851-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinoza R, Kusne S, Pasculle AW, Wada S, Fung J, Rakela J. Leuconostoc bacteremia after liver transplantation: another cause of vancomycin resistant gram-positive infection. Clin Transplant. 1997;11:322–324. [PubMed] [Google Scholar]
- 9.Bou G, Saleta JL, Sáez Nieto JA, Tomás M, Valdezate S, Sousa D, Lueiro F, Villanueva R, Jose Pereira M, Llinares P. Nosocomial outbreaks caused by Leuconostoc mesenteroides subsp mesenteroides. Emerg Infect Dis. 2008;14:968–971. doi: 10.3201/eid1406.070581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arico M, Janka G, Fischer A, Henter JI, Blanche S, Elinder G, Martinetti M, Rusca MP. Hemophagocytic lymphohistiocytosis. Report of 122 children from the International Registry. FHL Study Group of the Histiocyte Society. Leukemia. 1996;10:197–203. [PubMed] [Google Scholar]
- 11.Cappelli EA, Barros RR, Camello TC, Teixeira LM, Merquior VL. Leuconostoc pseudomesenteroides as a cause of nosocomial urinary tract infections. J Clin Microbiol. 1999;37:4124–4126. doi: 10.1128/jcm.37.12.4124-4126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tholpady SS, Sifri CD, Sawyer RG, Hazen KC, Pruett TL, Bonatti H. Leuconostoc pseudomesenteroides blood stream infection following liver transplantation. Ann Transplant. 2010;15:61–66. [PubMed] [Google Scholar]
- 13.Rodríguez J, Saavedra J, Fernández-Jurado A, Prados D. Leuconostoc pseudomesenteroides bacteremia. Sangre. 1999;44:82–83. (In Spanish) [PubMed] [Google Scholar]
- 14.Azendour H, Lahlou J, Massou S, Balkhi H, Haimeur C. Leuconostoc mesenteroides bacteremia. Ann Fr Anesth Reanim. 2008;27:457–458. doi: 10.1016/j.annfar.2008.03.013. (In French) [DOI] [PubMed] [Google Scholar]
- 15.Friedland IR, Snipelisky M, Khoosal M. Meningitis in a neonate caused by Leuconostoc sp. J Clin Microbiol. 1990;28:2125–2126. doi: 10.1128/jcm.28.9.2125-2126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacometti A, Ranaldi R, Siquini FM, Scalise G. Leuconostoc citreum isolated from lung in AIDS patient. Lancet. 1993;342:622. doi: 10.1016/0140-6736(93)91452-R. [DOI] [PubMed] [Google Scholar]
- 17.Golledge CL. Infection due to Leuconostoc species. Rev Infect Dis. 1991;13:184–185. doi: 10.1093/clinids/12.5.184. [DOI] [PubMed] [Google Scholar]
- 18.Green M, Wadowsky RM, Barbadora K. Recovery of vancomycin-resistant gram-positive cocci from children. J Clin Microbiol. 1990;28:484–488. doi: 10.1128/jcm.28.3.484-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handwerger S, Horowitz H, Coburn K, Kolokathis A, Wormser GP. Infection due to Leuconostoc species: Six cases and review. Rev Infect Dis. 1990;12:602–610. doi: 10.1093/clinids/12.4.602. [DOI] [PubMed] [Google Scholar]


