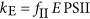
|
The rate of generation of an excited catalytic Chlorophyll a (Chl a) dimer in photosystem II (PSII*). It depends on irradiance (E), the availability of the ground state Chl a in PSII (PSII) and the absorbance cross-section factor fII that describes the efficiency of conversion of the externally available photon flux (E) into a volumetric rate of excitation |
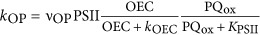
|
The rate of PQ reduction by PSII, that is, the rate of oxygenic photosynthetic electron transport. It depends on the availability of the excited catalytic Chl a dimer in PSII (PSII*), non-inhibited oxygen evolving complex (OEC) and oxidized plastoquinone (PQox). This process results in the formation of a highly reactive oxidized oxygen evolving complex (OECox), reduced plastoquinone (PQred) and regeneration of ground state Chl a in PSII (PSII) |
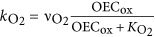
|
The rate of O2 release from the OEC, which depends on the availability of oxidized OEC (OECox) |
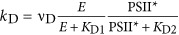
|
The rate of PSII degradation by photoinhibition. It depends on the availability of the excited catalytic Chl a dimer in PSII (PSII*) and the irradiance (E). To account for light-dependent efficiency of photoinhibition the rate saturates at high light intensities |
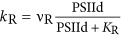
|
The rate of repair of the partially degraded, non-active PSII (PSIId) |
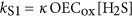
|
The rate of OECox inhibition by H2S. This rate depends on the availability of the intermediate formed during OEC oxidation (OECox) and yields OEC:H2S, which refers to H2S being bound to OECox
|
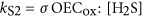
|
The rate of OECox release from OECox:H2S, that is, the rate of deinhibition |
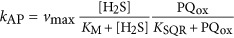
|
The rate of H2S oxidation coupled to PQox reduction by SQR, that is, the rate of anoxygenic photosynthetic electron transport. This process results in the formation of zero-valent sulfur and PQred
|
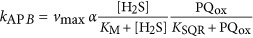
|
Assuming that a hypothetical additional sulfide-oxidizing enzyme (see ‘USO’ in kAP2) exists or that two SQRs exist that are expressed dependent on the light intensity (not shown), this rate is kAP and depends exclusively on the H2S concentration ([H2S]) and the availability of PQox
|
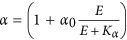
|
Assuming that the activity of SQR is directly regulated by the light intensity the maximum rate of H2S oxidation this rate is kAP_B. In this rate νmax, additionally depends on a factor α that increases with irradiance (E) |
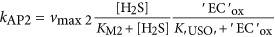
|
The rate of H2S oxidation coupled to the reduction of another electron transport chain component (‘EC’), such as oxidized cytochrome c, by a hypothetical sulfide oxidizing enzyme ‘USO’. This rate is only included in the model when assuming that the activity of SQR is not directly regulated by the light intensity (see description of kAP and kAP_B) |
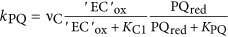
|
The rate of PQred oxidation coupled to the reduction of any electron transport chain component (‘EC’). This process results in the reformation of PQox, which is available again for the reduction by SQR or PSII |
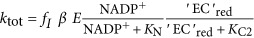
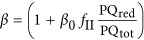
|
The rate of NADP+ reduction coupled to the oxidation of the unidentified electron transport component ‘EC’. This rate depends on the availability of reduced ‘EC’, NADP+, irradiance (IPSI in μmol photons m−2 s−1), and the absorbance cross-section factor fI that describes the efficiency of conversion of the externally available photon flux (E) into a volumetric rate of excitation in PSI. fI is increased by excitation energy transfer from PSII to PSI, which is described by the transfer factor β. β depends on the redox state of the plastoquinone pool where PQred refers to the reduced part of the total PQ pool (PQtot) |
| |
|
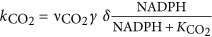
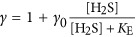
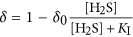
|
The rate of CO2 fixation coupled to NADPH oxidation, which depends on the maximum rate of CO2 reduction (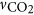 ) and the availability of NADPH. ) and the availability of NADPH. 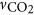 is enhanced when H2S available, which is described by the factor γ. At high H2S concentrations (KE<<KI) is enhanced when H2S available, which is described by the factor γ. At high H2S concentrations (KE<<KI) 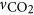 decreases again, which is described by the factor δ decreases again, which is described by the factor δ
|
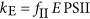
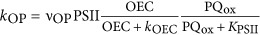
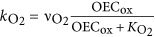
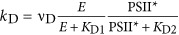
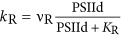
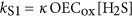
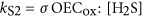
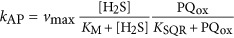
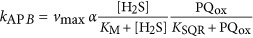
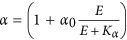
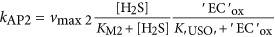
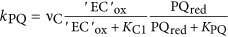
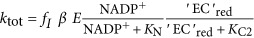
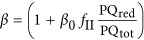
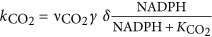
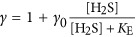
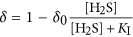
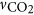 ) and the availability of NADPH.
) and the availability of NADPH. 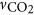 is enhanced when H2S available, which is described by the factor γ. At high H2S concentrations (KE<<KI)
is enhanced when H2S available, which is described by the factor γ. At high H2S concentrations (KE<<KI) 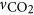 decreases again, which is described by the factor δ
decreases again, which is described by the factor δ
