Abstract
Introduction
As the population ages, the number of elderly patients with esophageal cancer increases. Esophageal cancer has a poor prognosis and is associated with decreased life quality.
Aim
To review the literature about the outcome of esophageal cancer in patients over 65.
Material and methods
Articles published between January 2006 and November 2016 in the PubMed/Medline and ResearchGate databases were reviewed. Nineteen retrospective studies were included.
Results
Six thousand seven hundred and twenty-nine patients over 65 were analyzed. Thirty-day mortality ranges from 3.2% to 8.1%. Overall 5-year survival rates range from 0% to 49.2%, and the median survival rate ranges from 9.6 to 108.2 months. The incidence of complications in the surgery group ranges from 27% to 69%. Chemoradiotherapy grade ≥ 3 toxicity was observed in 22–36% of patients.
Conclusions
Chronological age seems to have little influence on outcome of esophageal cancer. Open esophagectomy seems to be the mainstay of treatment for patients with esophageal cancer, regardless of age. There is still high mortality and morbidity involved in this procedure. To reduce them, some less invasive methods are being trialed.
Keywords: minimally invasive surgery, chemotherapy, esophageal cancer, outcomes, elderly, esophagectomy
Introduction
The progress in medicine, including the prolonged life expectancy, suggests that the number of older elderly patients with esophageal cancer will significantly increase in the coming years. This group of patients is very heterogeneous with regard to co-morbidity and physical reserve, while no clear guidelines of esophageal cancer management for the elderly are available. Regardless of the advance in surgical methods and chemoradiotherapy, the prognosis in this type of cancer remains poor. Despite the high incidence of this type of cancer among the elderly, no review relevant to geriatric patients is available.
Aim
The current study aimed to review the literature about the outcome of esophageal cancer in patients over 65.
Material and methods
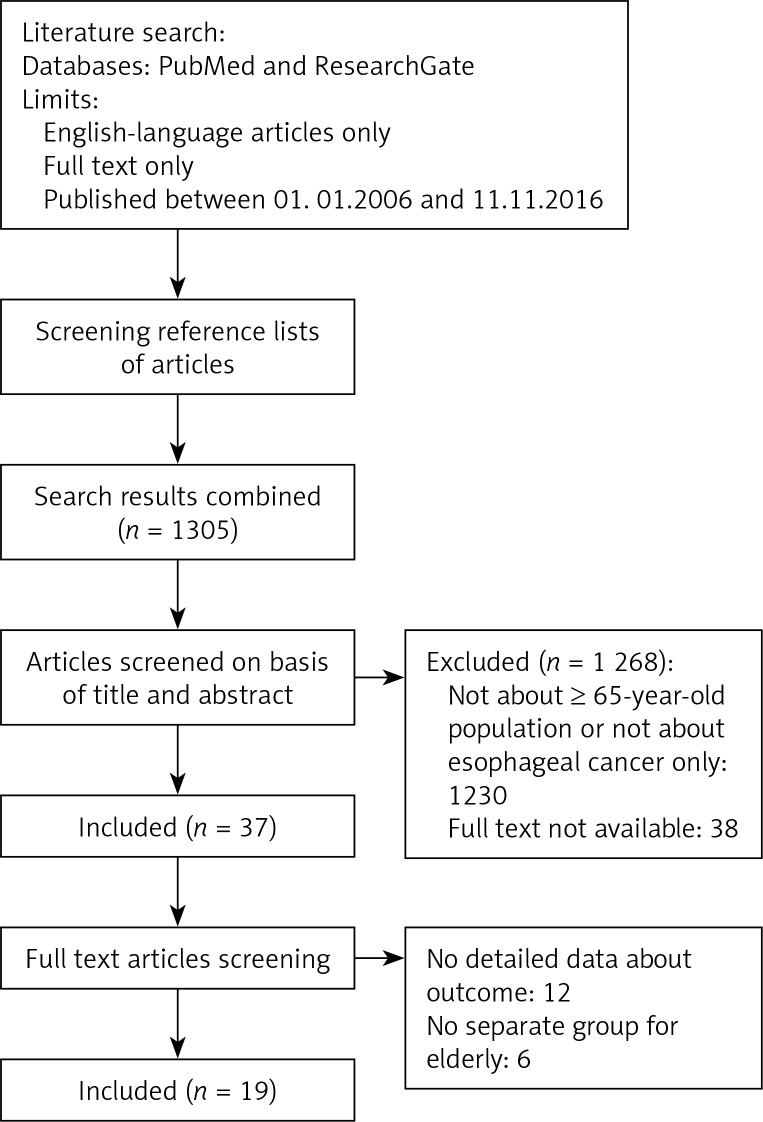
A systematic review of the literature was conducted using the PubMed/Medline and Research-Gate databases and by screening reference lists of articles. The databases were searched using the phrase “esophageal cancer” AND “elderly”. The titles, abstracts and full-text versions of studies published between January 1, 2006 and November 11, 2016 were sought out for inclusion in the review. Studies of populations aged over 65, treated for esophageal cancer, containing data about outcomes of the treatment (e.g. morbidity, mortality, survival rates), written in English, were included. Case reports, review papers and abstracts were excluded from the review. Finally 19 articles were chosen (Figure 1). Considering 13 studies, authors compared treatment outcome between specified groups of patients. Cummings et al. compared endoscopic treatment (ET) and open esophagectomy (OE) groups [1]. Li et al. compared OE and minimal invasive esophagectomy (MIE) groups [2]. Abrams et al. Tougeron et al. and compared OE and chemoradiotherapy (CRT) groups [3, 4]. Tougeron et al. presented the results in curative treatment, palliative treatment and best supportive care groups [5]. Tapias et al. analyzed 3 groups based on age. Two of them concerned patients aged over 65 years (≥ 70 and ≥ 80) [6]. Among 7 studies, two groups based on age were compared (younger vs. elderly) [6–12]. Only the groups consisting of patients ≥ 65 years were analyzed.
Figure 1.
Flow diagram
Results
Tables I and II include detailed information on population characteristics. Table III presents data on patients’ outcomes. All 19 articles were retrospective studies. In total, 6729 patients were included. Most of them were male (4888; 72.64%) while only 23.36% (1841 patients) were female. The authors used four different scales to assess pre-treatment performance status of patients. Seven of them used the American Society of Anesthesiologists (ASA) score, five used the Charlson Comorbidity Index and one used the Eastern Cooperative Oncology Group (ECOG) score. The percentage of patients with comorbidities was in the range 29–84%. One thousand six hundred and thirty-six (51.45%) patients suffered from adenocarcinoma and 1 439 (45.3%) from squamous cell carcinoma. The other cell types were rare (n = 105; 3.3%). Four hundred and eighty-two (47.6%) patients had a tumor located in the lower third of the esophagus, 394 (38.9%) in the middle third and 137 (13.5%) in the upper third. Considering all studies which present data about cancer stage, 167 (5.5%), 1565 (51.6%), 760 (25.1%), 445 (14.7%) and 95 (3.1%) patients were diagnosed with tumor stage 0, I, II, III and IV, respectively. Open esophagectomy was the most common treatment method (n = 2.023; 30.1%). Five hundred and sixty-nine (8.5%) patients received chemoradiotherapy, 268 (4%) endoscopic treatment and 65 (1%) minimally invasive esophagectomy. Thirty (0.5%) patients received palliative treatment consisting of photodynamic therapy (n = 1; 3.3%) or chemotherapy (n = 20; 66.7%). 666 (9.9%) patients received best supportive care instead of curative treatment. According to the analyzed publications, 30-day mortality ranges from 3.2% to 8.1%. Overall 5-year survival rates range from 0 to 49.2%, and the median survival rate ranges from 9.6 to 108.2 months. Twelve to seventy percent of patients who had undergone different types of therapy suffered from treatment-related complications. Considering CRT, grade ≥ 3 toxicity was observed in 22–36% of patients. The incidence of complications in the surgery group ranges from 27% to 69%. Medical complications occurred more often than surgical ones. The most common were cardiopulmonary ones, such as pneumonia and arrhythmia. The most important surgical complications were anastomotic leakage, wound infection and chylothorax.
Table I.
List of publications concerning esophageal cancer outcomes in the elderly and the most important data about the methodology of the publications
| Authors | Study period [years] | Number of patients (male/female) | Age [years] | Median age [years] | Type of treatment, n (%) | Follow-up period |
|---|---|---|---|---|---|---|
| Cummings et al. (OE group) | 1994–2011 | 893 (691/202) | > 66 | 74.3 ±5.4 | OE | 2 years |
| Liu, Huang et al. (≥ 70 y group) | 2001–2012 | 39 (31/8) | ≥ 70 | 75.1 ±3.6 | OE | Until death/until the end of research |
| Li et al. (OE group) | 2005– 2013 | 58 (44/14) | > 70 | 72 (70–85) | OE | Until death/until the end of research |
| Aydin et al. | 1998–2010 | 37 (13/24) | ≥ 70 | 74 ±3.7 (70–83) | OE | Until death/until the end of research |
| Pultrum et al. (≥ 70 y group) | 1991–2007 | 64 (52/12) | ≥ 70 | 74.5 | OE | Until death/until the end of research |
| Liu, Chen et al. (≥ 70 y group) | 1999–2007 | 29 (22/7) | > 70 | 75.2 ±3.6 | OE | Until death/until the end of research |
| Kosugi et al. (OE group) | 1992–2003 | 40 (38/2) | ≥ 70 | 77 (75–85) | OE | Until death/until the end of research |
| Abrams et al. (OE group) | 1991–2002 | 341 (257/84) | ≥ 65 | nd | OE | Until death/until the end of research |
| Internullo et al. | 1991–2006 | 108 (76/32) | ≥ 76 | nd | OE | Until death/until the end of research |
| Ruol et al. (≥ 70 y group) | 1992–2005 | 159 (124/35) | ≥ 70 | 73.1 (71.6–76.6) | OE | Until death/until the end of research |
| Ma et al. (≥ 70 y group) | 1990–2004 | 60 (51/9) | ≥ 70 | 73.1 ±3.9 | OE | 6 months |
| Mirza et al. (≥ 70 y group) | 1996–2010 | 46 (37/9) | ≥ 70 | nd | OE | Until death/until the end of research |
| Li et al. (MIE group) | 2005– 2013 | 58 (44/14) | > 70 | 72 (70–79) | MIE | Until death/until the end of research |
| Cummings et al. (ET group) | 1994–2011 | 255 (197/58) | > 66 | 77.5 ±6.4 | ET | 2 years |
| Kikuchi et al. (≥ 75 y group) | 2005–2011 | 13 (11/2) | ≥ 70 | 79 (76–87) | ESD | 7 days |
| Wakui et al. | 2003–2008 | 22 (19/3) | ≥ 75 | 79 (75–85) | CRT | Until death/until the end of research |
| Kosugi et al. (CRT group) | 1992–2003 | 24 (21/3) | ≥ 70 | 77 (75–85) | CRT | Until death/until the end of research |
| Abrams et al. (CRT group) | 1991–2002 | 389 (261/128) | ≥ 65 | nd | CRT | 10 years |
| Tougeron, Di Fiore et al. | 1994–2007 | 109 (90/19) | ≥ 70 | 74.4 ±3.7 (70–88) | CRT | Until death/until the end of research |
| Anderson et al. | 1996–2001 | 25 (14/11) | 65–70 (n = 2) ≥ 70 (n = 23) |
77 (66–88) | CRT | Until death/until the end of research |
| Tougeron et al. (curative treatment) | 1994–2007 | 151 (124/27) | ≥ 70 | 74.9 ±4.1 | Mucosectomy: 6 (4.0) PDT: 14 (9.3) Surgery: 13 (8.6) CRT: 111 (73.5) RT: 7 (4.6) |
Until death/until the end of research |
| Tougeron et al. (palliative treatment group) | 1994–2007 | 30 (27/3) | ≥ 70 | 74.2 ±4.0 | PDT: 1 (3.3) CT: 20 (66.7) |
Until death/until the end of research |
| Tougeron et al. (BSC group) | 1994–2007 | 101 (65/36) | ≥ 70 | 80.0 ±6.6* 78.2 ±5.8** |
PDT: 3 (4.3)* CT: 2 (2.9)* |
Until death/until the end of research |
| Tapias et al. (70–79 y group) | 2002–2011 | 124 (99/25) | 70–79 | 73.8 ±2.9 | MIE (n = 7) OE (n = 133) |
10 years |
| Tapias et al. (≥ 80 y group) | 2002–2011 | 16 (10/6) | ≥ 80 | 82.2 ±1.6 | MIE (n = 0) OE (n = 16) |
10 years |
| Steyerberg et al. | 1991–1999 | 3538 (2470/1068) | ≥ 65 | nd | Combinations of OE, CT and RT (n = 2973) BSC (n = 565) |
Until death/until the end of research |
ET – endoscopic treatment (ablation/endoscopic mucosal resection), OE – open esophagectomy, EMR – endoscopic mucosal resection, MIE – minimal invasive esophagectomy, ESD – endoscopic submucosal dissection, CRT – chemoradiotherapy, PDT – photodynamic therapy, RT – radiotherapy, CT – chemotherapy, BSC – best supportive care
patients without visceral metastasis/metastases
patients with visceral metastasis/metastases, nd – no data.
Table II.
List of publications concerning esophageal cancer outcomes in the elderly and the most important data about the population
| Authors | Histology, n (%) | Tumor stage, n (%) | Tumor site | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCC | AC | Other | 0 | I | II | III | IV | L1/3 | M1/3 | U1/3 | |
| Cummings et al. (OE group) | 261 (29.23) | 632 (70.77) | 0 (0) | 88 (9.9) | 805 (90.1) | 0 (0) | 0 (0) | 0 (0) | nd | nd | nd |
| Liu, Huang et al. (≥ 70 y group) | 30 (76.9) | 9 (23.1) | 0 (0) | 0 (0) | 0 (0) | 20 (51.3) | 14 (35.9) | 5 (12.8) | 17 (43.6) | 16 (41.0) | 6 (15.4) |
| Li et al. (OE group) | 58 (100) | 0 (0) | 0 (0) | 0 (0) | 3 (5.2) | 30 (51.7) | 25 (43.1) | 0 (0) | 6 (10.3) | 42 (72.4) | 10 (17.2) |
| Aydin et al. | 27 (73.0) | 6 (16.2) | 4 (10.8) | 0 (0) | 4 (10.8) | 19 (51.4) | 14 (37.8) | 0 (0) | 18 (48.6) | 17 (46.0) | 2 (5.4) |
| Pultrum et al. (≥ 70 y group) | 8 (13) | 56 (87) | 0 (0) | 0 (0) | 11 (17) | 25 (52) | 25 (39) | 3 (5) | 60 (94) | 4 (6) | 0 (0) |
| Liu, Chen et al. (≥ 70 y group) | 26 (89.7) | 3 (10.3) | 0 (0) | 0 (0) | 0 (0) | 14 (48.2) | 11 (37.9) | 4 (13.8) | 13 (44.8) | 11 (37.9) | 5 (17.2) |
| Kosugi et al. (OE group) | 40 (100) | 0 (0) | 0 (0) | 0 (0) | 11 (27.5) | 15 (37.5) | 11 (27.5) | 3 (7.5) | 18 (45.0) | 19 (47.5) | 3 (7.5) |
| Abrams et al. (OE group) | 94 (27.5) | 213 (62.5) | 34 (10.0) | 0 (0) | 177 (51.9) | 164 (48.1) | 0 (0) | 0 (0) | nd | nd | nd |
| Internullo et al. | 26 (24) | 80 (74) | 2 (1.8) | 6 (5.5) | 29 (26.8) | 25 (23.1) | 29 (26.7) | 19 (17.5) | 93 (86.1) | 13 (12) | 2 (1.8) |
| Ruol et al. (≥ 70 y group) | 77 (48.4) | 77 (48.4) | 5 (3.2) | 5 (3.2) | 20 (12.7) | 71 (44.2) | 50 (31.9) | 11 (7.0) | 94 (59.1) | 37 (23.3) | 28 (17.6) |
| Ma et al. (≥ 70 y group) | 53 (88.3) | 4 (6.7) | 3 (5.0) | 1 (1.7) | 2 (2.2) | 9 (15.0) | 45 (75.0) | 3 (5.0) | 16 (26.7) | 32 (53.3) | 10 (16.7) |
| Mirza et al. (≥ 70 y group) | 45 (97.83) | 1 (2.17) | 0 (0) | nd | nd | nd | nd | nd | nd | nd | nd |
| Li et al. (MIE group) | 58 (100) | 0 (0) | 0 (0) | 0 (0) | 4 (6.9) | 31 (53.4) | 23 (39.7) | 0 (0) | 8 (13.8) | 44 (75.9) | 6 (10.3) |
| Cummings et al. (ET group) | 78 (31) | 177 (69) | 0 | 60 (23.5) | 195 (76.5) | 0 (0) | 0 (0) | 0 (0) | nd | nd | nd |
| Kikuchi et al. (≥ 75 y group) | 13 (100) | 0 (0) | 0 (0) | nd | nd | nd | nd | nd | 1 (8) | 9 (69) | 3 (23) |
| Wakui et al. | 22 (100) | nd | nd | 0 (0) | 3 (13.64) | 6 (27.27) | 12 (54.55) | 1 (4.55) | nd | 10 (46) | nd |
| Kosugi et al. (CRT group) | 24 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (4.2) | 14 (58.3) | 7 (29.2) | 2 (8.4) | 3 (12.5) | 15 (62.5) | 6 (25.0) |
| Abrams et al. (CRT group) | 209 (53.7) | 141 (36.3) | 39 (10.0) | 0 (0) | 232 (59.6) | 157 (40.4) | 0 (0) | 0 (0) | nd | nd | nd |
| Tougeron, Di Fiore et al. | 77 (70.6) | 28 (25.7) | 4 (3.7) | 0 (0) | 2 (1.8) | 50 (45.9) | 46 (42.2) | 6 (5.5) | 52 (47.7) | 36 (33.3) | 21 (19.3) |
| Anderson et al. | 13 (52) | 12 (48) | 0 (0) | 0 (0) | 0 (0) | 8 (32) | 17 (68) | 0 (0) | nd | nd | nd |
| Tougeron et al. (curative treatment) | 103 (70.1) | 44 (29.9) | 0 (0) | 0 (0) | 24 (15.9) | 61 (40.4) | 51 (33.8) | 7 (4.6) | 80 (60.0) | 44 (29.1) | 27 (17.9) |
| Tougeron et al. (palliative treatment group) | 17 (56.7) | 12 (40.0) | 1 (3.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 30 (100) | 19 (63.3) | 9 (30.0) | 2 (6.7) |
| Tougeron et al. (BSC group) | 63 (67.02) | 27 (28.72) | 4 (4.26) | 0 (0) | 3 (4.3) | 7 (10.1) | 18 (26.1) | 1 (1.4) | 52 (51.49) | 33 (32.67) | 16 (15.84) |
| Tapias et al. (70–79 y group) | 15 (12.1) | 100 (80.7) | 9 (7.2) | 7 (6.2) | 34 (30.1) | 29 (25.7) | 43 (38.1) | 0 (0) | nd | nd | nd |
| Tapias et al. (≥ 80 y group) | 2 (12.5) | 14 (87.5) | 0 (0) | 0 (0) | 5 (35.7) | 5 (35.7) | 4 (28.6) | 0 (0) | nd | nd | nd |
| Steyerberg et al. | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
L1/3 – lower third, M1/3 – middle third, U1/3 – upper third, SCC – squamous cell carcinoma, AC – adenocarcinoma, nd – no data.
Table III.
List of publications concerning esophageal cancer outcomes in the elderly and the most important data about the outcomes
| Authors | Pre-treatment comorbidity assessment score | Mortality, n (%) | All complications/toxicity, n (%) | Overall survival rates (%) | Median overall survival [months] | Recurrent disease, n (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 5 years | ||||||
| Cummings et al. (OE group) | Charlson/Deyo comorbidity index | 36 (4)2 | 265 (30) | nd | 71 (AC) 60 (SCC) |
nd | nd | nd | 139 (16) |
| Liu, Huang et al.
(≥ 70 y group) |
nd | 3 (7.7)5 | 18 (46.1) | nd | 33.3 | 0 | nd | 15.8 | nd |
| Li et al. (OE group) | nd | 5 (8.6)5 | 35 (60.3) | nd | nd | nd | nd | 22 ±3.4 | nd |
| Aydin et al. | nd | 3 (8.1)1 | 16 (43.2) | 70.3 | nd | 31 | 21.4 | 28.7 | 6 (16.2) |
| Pultrum et al. (≥ 70 y group) | ASA | 7 (11)3 | 44 (69) | 70 | nd | nd | 33 | 26 (range: 0–199) | 27 (42) |
| Liu Chen et al. (≥ 70 y group) | ECOG | 3 (10.3)4 | 15 (51.7) | nd | 20 | 3 | 0 | 12.1 (95% CI: 8.6–15.6) | nd |
| Kosugi et al. (OE group) | ASA | 2 (5)1 | 26 (65.0) | 77.5 | nd | 37.3 | 24.0 | 108.2 (range: 32.5–138.9) | nd |
| Abrams et al. (OE group) | Charlson/Klabunde comorbidity index | 24 (7.7)1 | nd | nd | nd | 53.1 | 44.9 | nd | nd |
| Internullo et al. | ASA | 8 (7.4)5 | 56 (51.9) | nd | nd | nd | 35.7 | 28.5 (range: 0.1–149.5) | (33.3) |
| Ruol et al. (≥ 70 y group) | ASA | 3 (1.9)1 3 (1.9)4 |
78 (49.1) | nd | nd | nd | 35.4 | 17.9 (range: 9.2–44.4) | nd |
| Ma et al. (≥ 70 y group) | nd | 2 (3.3)4 | nd | nd | nd | nd | nd | nd | nd |
| Mirza et al. (≥ 70 y group) | ASA | nd | nd | nd | nd | nd | nd | 10.8 (range: < 1 month to 8.3 years) | 21 (45.65) |
| Li et al. (MIE group) | nd | 2 (3.4)5 | 22 (37.9) | nd | nd | nd | nd | 39 ±8.9 | nd |
| Cummings et al. (ET group) | Charlson/Deyo comorbidity index | nd | 30 (12) | nd | 84 (AC) 76 (SCC) |
nd | nd | nd | 32 (13) |
| Kikuchi et al. (≥ 75 y group) | ASA | 0 (0)5 | 13 (27) | nd | nd | nd | nd | nd | nd |
| Wakui et al. | nd | 4 (18.2)5 | 14 (70) | 44.3 ±10.8 | 34.5 ±10.4 | 15.9 ±10.8 | nd | 23.8 (range: 8.3–53.6) | 11 (55) |
| Kosugi et al. (CRT group) | ASA | 5 (21)6 | nd | 60.9 | nd | 17.4 | 11.6 | 72.5m (range: 12.4–95.3) | 1 (4.1) |
| Abrams et al. (CRT group) | Charlson/Klabunde comorbidity index | nd | nd | nd | nd | 23.9 | 13.9 | nd | nd |
| Tougeron, Di Fiore et al. | Charlson score | 2 (2.2)5 | 62 (56.9) | 56.9 | 19.27 | 6.4 | 15.2 ±2.8 | 31 (28.4) | |
| Anderson et al. | Charlson score | 0 (0)5 | 9 (36) | 80 | 64 | nd | nd | 35 (3–66) | nd |
| Tougeron et al. (curative treatment) | Charlson score | nd | nd | nd | nd | nd | nd | 17.8 ±1.5* 5.5 ±2.0** |
35 (23.17) |
| Tougeron et al. (palliative treatment group) | Charlson score | nd | nd | nd | nd | nd | nd | 6.7 ±2.1 | 7 (23.33) |
| Tougeron et al. (BSC group) | Charlson score | nd | nd | nd | nd | nd | nd | 5.5 ±2.0* 1.8 ±0.4** |
18 (17.82) |
| Tapias et al. (70–79 y group) | nd | 4 (3.2)1 7 (6.1)3 |
77 (62.1) | nd | nd | nd | 41.7 | nd | nd |
| Tapias et al. (≥ 80 y group) | nd | 1 (6.3)1 2 (14.3)3 |
14 (87.5) | nd | nd | nd | 49.2 | nd | nd |
| Steyerberg et al. | Charlson score | nd | nd | 42 | 24 | nd | 11 | 9.63 (95% CI: 9.2–10) | nd |
30-day mortality
60-day mortality
90-day mortality
in-hospital mortality
no data
treatment-related mortality
patients without visceral metastasis/metastases
patients with visceral metastasis/metastases, nd – no data.
Discussion
Histology type, location and stage
All publications included detailed information about the cancer histology type. Most of them provide information about tumor location and stage. Three studies described connection between cancer histology type and outcome. Cummings et al. noted that patients with adenocarcinoma (AC) who underwent open esophagectomy (OE) or endoscopic treatment (ET) had poorer 2-year survival compared to squamous cell carcinoma (SCC) patients (60% vs. 76%; p < 0.01) [1]. However, Abrams et al. reported that SCC patients were refused surgery more often, but AC patients treated with chemoradiotherapy (CRT) had worse overall and disease-specific survival than the SCC group. They noted that there is a difference in response to CRT between AC and SCC. Considering their results, older patients with AC can benefit from CRT more than SCC patients, but the protocol of chemoradiotherapy is not reported in the study. It is believed that this study may be underpowered to reveal such an advantage of CRT for SCC patients [3]. Anderson et al. found no significant difference in 2-year survival between AC and SCC groups treated with CRT consisting of 5-FU, mitomycin and radiation, but this can be explained by the small cohort size (25 patients) [13]. Comparing these results, it is difficult to determine which cancer type has a better prognosis among the elderly, because the conclusions of the studies are conflicting. More advanced tumor stage is a well-known factor of worse prognosis. It has also been confirmed by two studies, which revealed poorer overall survival among elderly patients with a more advanced cancer stage (I/II stage HR = 0.052; 95% CI: 0.005–0.039) [15], (HR = 1.63; 95% CI: 1.32–2.00) [7]. Another study showed that CRT patients diagnosed with stage I had higher disease-specific mortality than the group with stage II tumors. Surprising though it may be, in this study patients with more advanced disease were more likely to receive esophagectomy as a first-line therapy [3]. Two authors found no difference in overall survival considering groups based on tumor stage [4, 8]. However, this was not elaborated on in the discussion. Aydin et al. found that histology type, tumor location and tumor stage were not prognostic factors for treatment-related complications and mortality, but only 37 patients were included in their study [14].
Comorbidities
Performance status was assessed to evaluate individual risk of therapy, and to decide the treatment approach. Ruol et al. noted that, compared to the younger population, the elderly were excluded from surgery more often because of comorbidities (40% vs. 20%) [9]. Two papers mentioned “decreased functional body reserves” as a reason why elderly patients were considered unfit for surgery, but no detailed definition of decreased body reserve and assessment of frailty syndrome was reported [7, 15]. Three studies noted a significant impact of comorbidities on long-term survival [4, 7, 16]. Liu et al. identified poorer pulmonary function and limited functional reserve as a risk factor for higher mortality and morbidity after OE [7]. Among the articles, the usefulness of performance status scores was discussed. Tougeron et al. have shown that the Charlson score can be used as a prognostic factor for median overall survival (13.9 ±3.6 months Charlson score ≤ 2 vs. 4.1 ±2.6 months Charlson score > 2; HR = 2.1, 95% CI: 1.0–4.5; p = 0.046). Patients with a score ≥ 1 who underwent chemoradiotherapy were more likely to experience grade 2 or more toxicity (76.5% vs. 51.2%, p = 0.02) and chemotherapy delay (66.7% vs. 39.5%, p = 0.01) [4]. Steyerberg et al. described poorer survival in patients with a Charlson score ≥ 2 [16]. Pultrum et al. used the ASA score to assess patients, and did not consider it as a prognostic factor, but found that cardiovascular comorbidity among the elderly was a risk factor for postoperative comorbidity [8]. Tougeron et al. found no prognostic value of the Charlson score [5].
Effect of age
Eight studies, concerning surgical treatment, compared populations based on age. There was no study on chemoradiotherapy (CRT) comparing such groups. Only three papers have shown a significant difference in outcomes. Median survival described in one study differed in elderly and younger patients (151–306 days vs. 350–944 days) [16]. Moreover, there was a significant difference in the overall complication rate (53.6% (< 70 years) vs. 62.1% (70–79 years) vs. 87.5 (≥ 80 years); p = 0.011) [6]. Another study showed a significant increase in cardiac and pulmonary complications in patients aged ≥ 70 (pulmonary: 43.3% vs. 28.1%: p = 0.01; cardiac: 28.3% vs. 19.8%, p = 0.001), but no difference in the overall complication rate [10]. Age ≥ 70 was associated with longer intensive care unit stay with a median of 7 days (range: 1–64) in elderly versus younger patients, with a median of 3 days (range: 1–56) (95% CI: –9.95 to –1.86; p = 0.005) [8]. The report of Tapias et al. was the only study to show that elderly patients treated with less intensive radiotherapy or chemoradiotherapy had longer overall survival than younger patients (15.8 months vs. 13.7 months). The authors suggest that it can support the thesis that the tumor growth and metastatic spread become slower with aging. Considering the advantages of advanced age, another study showed decreased incidence of anastomotic stricture in the elderly group (OR = 0.99; p = 0.574). The authors postulated that the attenuated inflammatory response in the elderly leads to less collagen deposition and fibrosis [6]. Liu et al. observed longer survival time in the elderly treated with less intensive chemotherapy following esophagectomy in comparison to younger ones, but the results were statistically insignificant (median survival time 15.8 m vs. 13.7 m; p = 0.44). The other studies also found no significant difference in mortality, morbidity or long-term survival in younger and older group [8, 9, 11, 17].
Methods of treatment
Due to the retrospective character of studies, little is known about the process of therapeutic decision making. Two studies have shown a tendency for less invasive procedures to be conducted in older than in younger patients. Older age was associated with more frequent use of ET instead of OE [1]. Moreover, elderly patients were more likely to undergo transhiatal than transthoracic esophagectomy compared to the younger group [18]. Carcinoma in situ was considered as an indication for ET [1, 5]. One study considered advanced disease (≥ T3 and unresectable nodes) as a reason for refusing surgery [18]. Although the management of esophageal cancer in the elderly population is still discussed, esophagectomy seems to be the mainstay of treatment for patients with esophageal cancer [12]. Regardless of development of surgical techniques, there are still high mortality and morbidity involved in this procedure [5, 12]. Accordingly, some less invasive methods of treatment such as minimal invasive esophagectomy (MIE) or endoscopic treatment are being trialed [1, 2, 11]. Chemoradiotherapy alone is also considered a promising treatment method in some cases [5, 13, 16]. Cummings et al. observed decreased mortality (HR = 0.51; 95% CI: 0.36–0.73) and 2-year survival (HR = 0.61; 95% CI: 0.45–0.85) in the ET group (compared to OE), but only patients with early stage tumor (0, I) were operated on in such a way. Li et al. reported a significantly lower rate of overall complications in the MIE group than in the OE group (37.9 vs. 60.3, p = 0.016). In this study patients with stages I and II were included.
Kikuchi et al. observed no mortality in their study on endoscopic submucosal dissection, but it also pertains only to early stages of cancer (tumor diameter < 2 cm) and can be explained with non-invasive character of the procedure [11].
Quality of life
Unfortunately, no study has addressed the quality of life. Some complications such as esophageal stricture, vocal cord palsy or a need for tracheostomy can provide some data on this matter, but they cannot substitute detailed psychological analysis [19, 20].
Conclusions
Chronological age seems to have little influence on outcome of esophageal cancer treatment [7, 9, 11, 12, 14, 17]. Therefore, advanced age should not be considered as a contraindication for esophagectomy, but the risk should be evaluated individually. Currently, no study takes into consideration detailed geriatric assessment identifying the frailty state of the patient. There is also no study showing outcomes reported by patients that would present to the physicians the older patients’ view of the treatment process. Therefore, there is a great need for well-designed prospective studies including full geriatric assessment.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cummings LC, Kou TD, Schluchter MD, et al. Outcomes after endoscopic versus surgical therapy for early esophageal cancers in an older population. Gastrointest Endosc. 2016;84:232–40. doi: 10.1016/j.gie.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Shen Y, Tan L, et al. Is minimally invasive esophagectomy beneficial to elderly patients with esophageal cancer? Surg Endosc. 2015;29:925–30. doi: 10.1007/s00464-014-3753-x. [DOI] [PubMed] [Google Scholar]
- 3.Abrams JA, Buono DL, Strauss J, et al. Esophagectomy compared to chemoradiation for early stage esophageal cancer in elderly. Cancer. 2009;115:4924–33. doi: 10.1002/cncr.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tougeron D, Di Fiore F, Thureau S, et al. Safety and outcome of definitive chemoradiotherapy in elderly patients with esophageal cancer. Br J Cancer. 2008;99:1586–92. doi: 10.1038/sj.bjc.6604749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tougeron D, Hamidou H, Scotte M, et al. Esophageal cancer in the elderly: an analysis of the factors associated with treatment decisions and outcomes. BMC Cancer. 2010;10:510. doi: 10.1186/1471-2407-10-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapias LF, Muniappan A, Wright CD, et al. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg. 2013;95:1741–8. doi: 10.1016/j.athoracsur.2013.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Huang W, Chen C, et al. Radical esophagectomy in elderly patients with esophageal cancer. FJS. 2015;48:121–7. [Google Scholar]
- 8.Pultrum BB, Bosh DJ, Nijsten MWN, et al. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol. 2010;17:1572–80. doi: 10.1245/s10434-010-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg. 2007;133:1186–92. doi: 10.1016/j.jtcvs.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 10.Ma JY, Wu Z, Zhao YF, et al. Clinicopathologic characteristics of esophagectomy for esophageal carcinoma in elderly patients. World J Gastroenterol. 2006;12:1296–9. doi: 10.3748/wjg.v12.i8.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi O, Mouri H, Matsueda K, Yamamato H. Endoscopic submucosal dissection for treatment of patients aged 75 years and over with esophageal cancer. ISRN Gastroenterol. 2012;2012:671324. doi: 10.5402/2012/671324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirza A, Pritchard S, Welch I, et al. Is surgery in the elderly for oesophageal cancer justifiable? Results from a single centre. ISRN Surg. 2013;2013:609252. doi: 10.1155/2013/609252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson SE, Minsky BD, Bains M, et al. Combined modality chemoradiation in elderly oesophageal cancer patients. Br J Cancer. 2007;96:1823–7. doi: 10.1038/sj.bjc.6603821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aydin Y, Eroglu A, Turkyilmaz A, Gundogdu B. The effectiveness of transthoracic surgical procedures in elderly patients with esophageal cancer. Turk Gogus Kalp Dama. 2012;20:835–9. [Google Scholar]
- 15.Wakui R, Yamashita H, Okuma K, et al. Esophageal cancer: definitive chemoradiotherapy for elderly patients. Dis Esophagus. 2010;23:572–9. doi: 10.1111/j.1442-2050.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- 16.Steyerberg EW, Neville B, Weeks JC, Earle CC. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2007;25:2389–96. doi: 10.1200/JCO.2006.09.7931. [DOI] [PubMed] [Google Scholar]
- 17.Liu HC, Chen YC, Chen CH, Chen YJ. Esophagectomy in elderly patients with esophageal cancer. Int J Gerontol. 2010;4:176–9. [Google Scholar]
- 18.Kosugi S, Sasamoto R, Kanda T, et al. Retrospective review of surgery and definitive chemoradiotherapy in patients with squamous cell carcinoma of the thoracic esophagus aged 75 years or older. J Clin Oncol. 2009;39:360–6. doi: 10.1093/jjco/hyp030. [DOI] [PubMed] [Google Scholar]
- 19.Internullo E, Moons J, Nafteux P, et al. Outcome after esophagectomy for cancer of the esophagus and GEJ in patients over 75 years. Eur J Cardiothorac Surg. 2008;33:1096–104. doi: 10.1016/j.ejcts.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Orringer M. Age does not preclude an esophagectomy… If only it were that simple. Ann Surg Oncol. 2010;17:1487–9. doi: 10.1245/s10434-010-0989-0. [DOI] [PubMed] [Google Scholar]