Table 1.
| Compound number |

|
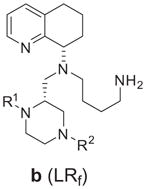
|
MAGI HIV1IIIB IC50a,b (μM) | SDF-1 Ca2+ Flux CEM-1 IC50a (μM) | |
|---|---|---|---|---|---|
| R1 | R2 | (*)-R/S | |||
| 68a | H | H | R | 0.31 | 0.255 |
| 68b | H | H | S | 1.1 | 1.2 |
| 52a/b | H | Ph | Mix. (1:1) | 0.72 | 0.13 |
| 48a | H | CH2Ph | R | 0.15 | 0.23 |
| 48b | H | CH2Ph | S | 0.03 | 0.19 |
| 49a | H | C(O)Ph | S | 0.06 | 0.002 |
| 49b | H | C(O)Ph | R | 0.02 | 0.006 |
| 69a | C(O)Ph | H | R | >100 | — |
| 69b | C(O)Ph | H | S | >100 | — |
| 50a/b | H |
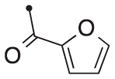
|
Mix. (2:1) | 0.21 | — |
| 50b | H | R | 0.15 | 0.035 | |
| 53a | H | CO2CH2Ph | S | 0.34 | 0.018 |
| 53b | H | CO2CH2Ph | R | 1.35 | — |
| 51a | H | SO2Ph | S | 0.05 | 0.002 |
| 51b | H | SO2Ph | R | 0.02 | 0.023 |
| 54a | H |
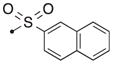
|
S | 4.6 | 0.071 |
| 55a | H |
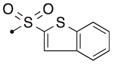
|
S | 3.44 | 0.039 |
| 56a | H |
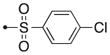
|
S | 0.05 | 0.002 |
| 57a | H | C(O)NHPh | S | 0.35 | 21 |
All assays were performed in duplicate.
The cytotoxicities (TC50’s) for all compounds were greater than 10 μM.
