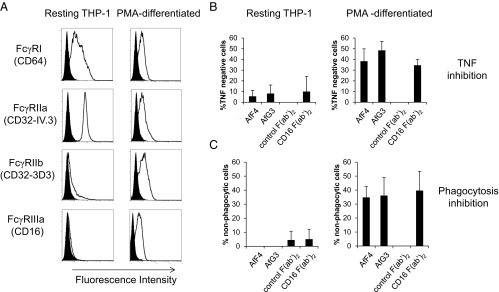
Fig. 5.
Affimer proteins are effective inhibitors of FcγRIIIa-dependent functions. (A) FACS profiles for resting monocytic THP-1 cells (Left) and PMA-differentiated, macrophage-like THP-1 cells (Right). In each case, the specific antibody staining is shown as an unfilled distribution, and the isotype control staining is shown as a filled distribution. The high-affinity FcγRIa (CD64) is reduced in differentiated THP-1 cells, along with the activatory FcγRIIa (CD32-IV.3). The inhibitory FcγRIIb is up-regulated in differentiated cells along with FcγRIIIa. (B) Affimer proteins were more effective in blocking HAG-induced TNF release in differentiated cells (Right) than in undifferentiated cells (Left), confirming their specificity for FcγRIIIa. This was represented as an increase in the percentage of TNF− cells. Blocking F(ab′)2 fragments against FcγRIIIa inhibited TNF production only in differentiated THP-1 cells. F(ab′)2 fragments against FcγRIII (CD16) were far more effective in differentiated cells, reflecting the differences in FcγRIIIa expression shown in A. F(ab′)2 fragments from preimmune serum had no effect on TNF production. (C) Both Affimer proteins were as effective as F(ab′)2 fragments in reducing phagocytosis of IgG opsonized E. coli in differentiated THP-1 cells.