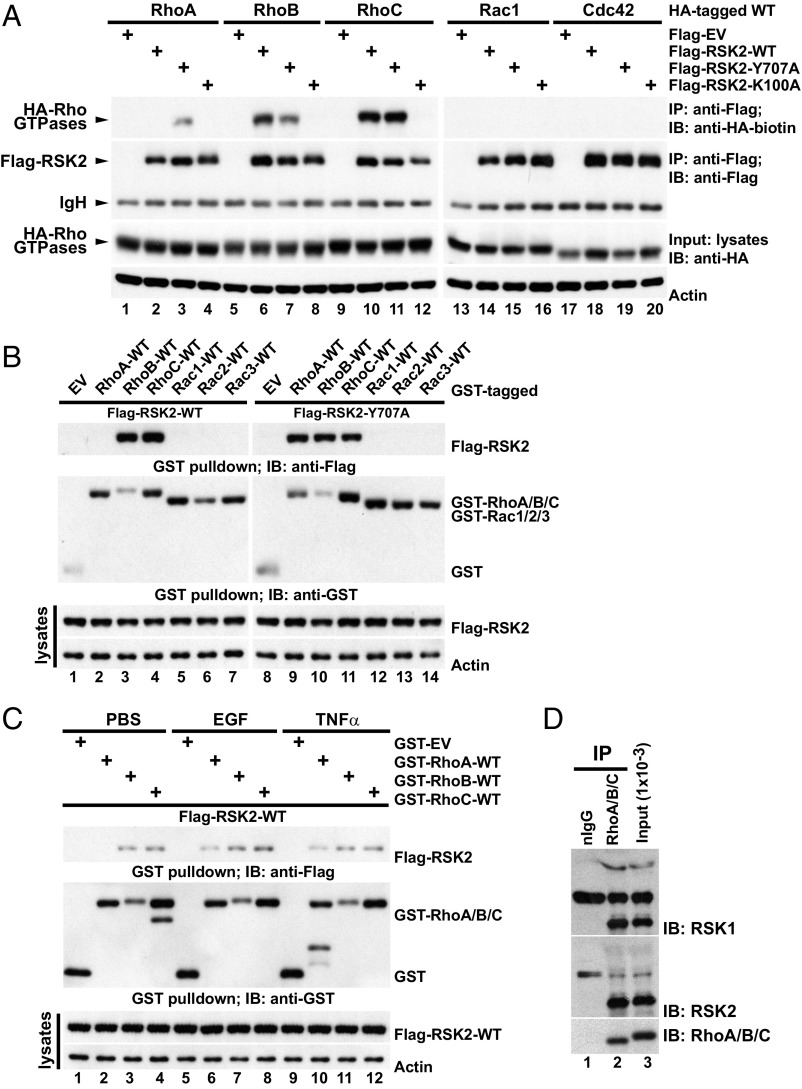
Fig. 2.
RSK2 forms a complex with RhoA GTPases. (A) U87MG cells transfected with the indicated Flag-RSK2 and wild-type (WT) HA-Rho GTPase constructs and serum starved. Lysates were subjected to anti–Flag-RSK2 immunoprecipitation (IP) and the coprecipitated HA-tagged Rho GTPases were detected by immunoblotting with biotinylated anti-HA antibody. The IP efficiency of Flag-RSK2 proteins and the equal loading of HA-Rho GTPases were determined. A representative of seven independent experiments is shown. (B) U87MG cells expressing GST-tagged WT-Rho GTPases together with Flag-RSK2-WT or Flag-RSK2-Y707A and serum starved. GST-Rho GTPases were recovered by GST pulldown, and coprecipitated Flag-RSK2 proteins were detected by immunoblotting with anti-Flag antibody. The precipitated GST-fused protein and equal loading of the Flag-RSK2 proteins are shown. The results shown are representative of three independent experiments. (C) U87MG cells were transfected with Flag-RSK2-WT and the indicated GST-Rho constructs and serum starved before stimulation with EGF (100 ng/mL, 5 min) or TNFα (50 ng/mL, 15 min). Lysates were subjected to GST pulldown. Coprecipitated Flag-RSK2 proteins were detected by immunoblotting with anti-Flag monoclonal antibody, while recovered GST-Rho proteins were determined by anti-GST immunoblotting. A representative of four independent experiments is shown. (D) U87MG cell lysate was subjected to immunoprecipitation with anti-RhoA/B/C antibody and bound proteins were recovered by incubation with protein-G Sepharose. The presence of coprecipitated RSK1/2 was determined by immunoblotting with RSK1 or -2 antibodies. The input levels of RSK1/2 and the immunoprecipitated Rho proteins are shown. nIgG, normal rabbit control IgG. A representative of four independent experiments is shown.