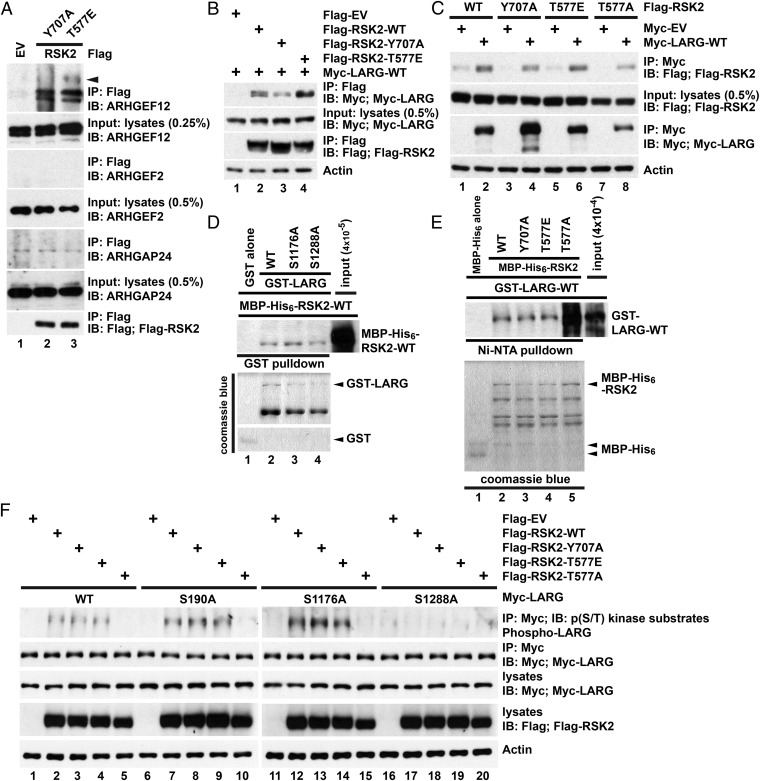
Fig. 4.
RSK2 forms a signaling complex composed of Rho GTPases and the RhoGEF, LARG. (A) Active RSK2 precipitates endogenous LARG. A total of 10 mg of U87MG cell lysates with overexpressed Flag-RSK2-Y707A or Flag-RSK2-T577E was incubated with anti-Flag antibody at 4 °C overnight and bound proteins were detected by immunoblotting with indicated antibodies. The arrow indicates possible phosphorylated LARG. (B and C) Association of RSK2 and LARG. U87MG cells were transfected with indicated Flag- or Myc-tagged constructs and serum starved for 24 h before lysate preparation. Flag (C)- or Myc (D)-tagged proteins were immunoprecipitated by anti-Flag or -Myc antibodies, respectively. Bound proteins were detected by immunoblotting with anti-Flag (RSK2) or -Myc (LARG) antibodies. Results are representative of three independent experiments. (D and E) RSK2 interacts directly with LARG. GST-LARG proteins and MBP-His6-RSK2-WT were incubated with either glutathione Sepharose (D) or Ni-NTA His-Bind resin (E). Bound MBP-His6-RSK2-WT (D) or GST-LARG (E) was determined by immunoblotting. (F) RSK2 phosphorylates LARG on Ser1288 residue. U87MG cells were cotransfected with Flag-RSK2 constructs and the indicated Myc-LARG constructs. The Myc-LARG proteins were immunoprecipitated after serum starvation for 24 h. LARG phosphorylation was determined by immunoblotting anti-Myc immunoprecipitates with a phospho-AKT substrate-specific antibody. Results are representative of three independent experiments.