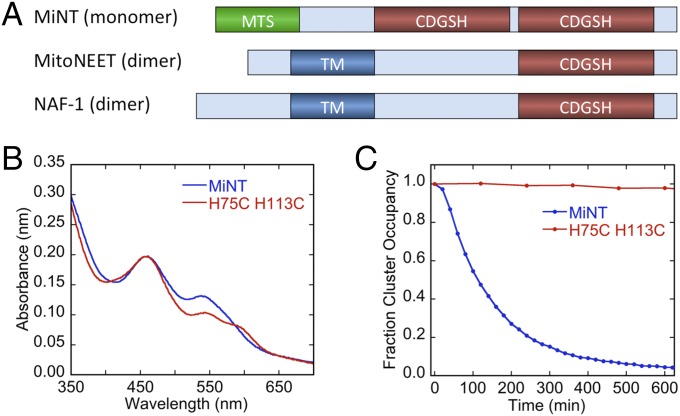
Fig. 1.
Domain organization of the NEET proteins and mutation of the cluster-coordinating His ligand of MiNT. (A) Domain organization. All members of the NEET family contain at least one CDGSH domain that binds a [2Fe-2S] cluster. MiNT has two CDGSH cluster-binding domains in a single polypeptide chain, while mNT and NAF-1 each have one domain per chain. MiNT lacks a transmembrane domain, but contains a classic cleavable mitochondrial targeting sequence. (B and C) Mutation of the cluster-coordinating His ligand to Cys stabilizes the [2Fe-2S] cluster. (B) UV-Vis absorption spectra of MiNT wild-type and H75C/H113C mutant proteins. (C) Relative stabilities of the [2Fe-2S] clusters of wild-type and H75C/H114C mutant MiNTs were measured by monitoring the decrease in absorbance of the 458-nm peak at pH 8.0 and 37 °C over time. Stability of the H75C/H113C mutant is greatly increased relative to wild-type MiNT under these conditions.