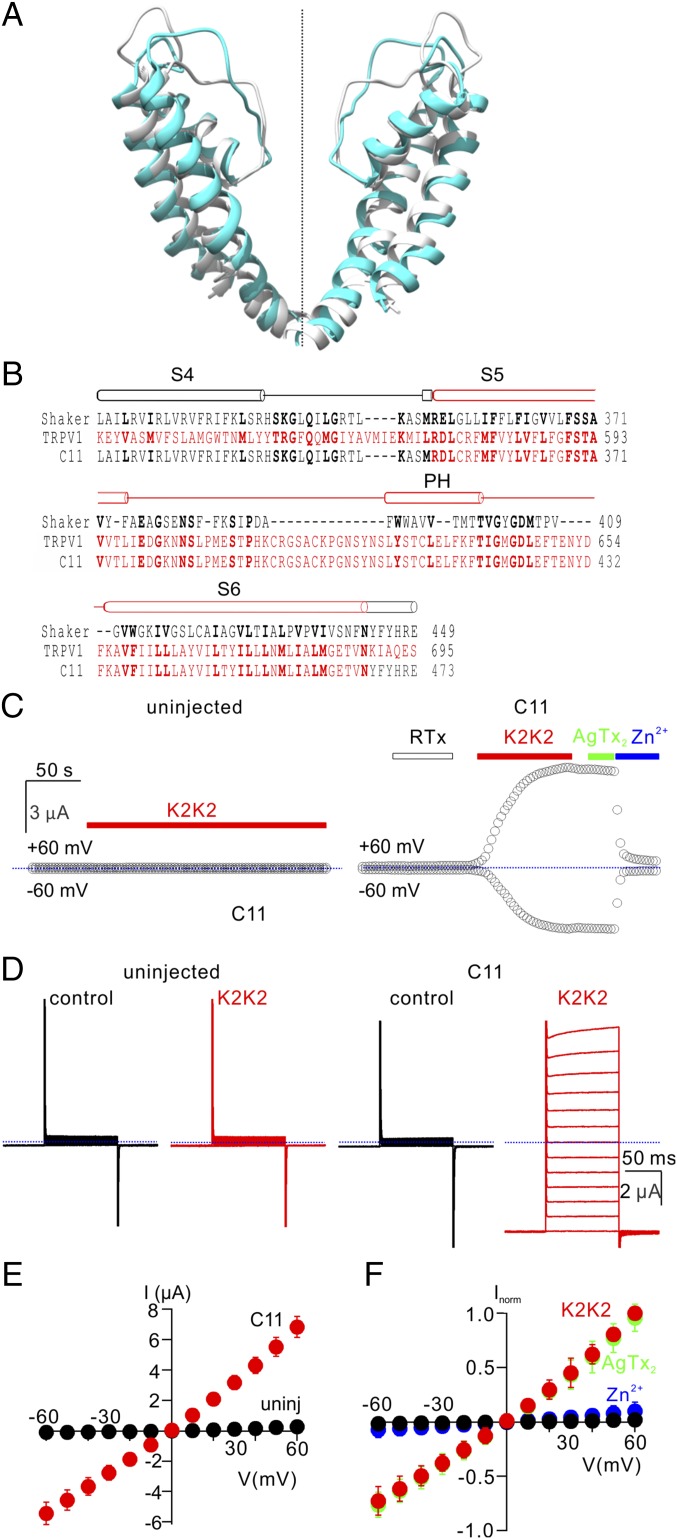
Fig. 1.
Transfer of the pore domain from TRPV1 into the Shaker Kv channel. (A) Backbone superposition of the pore domains of the Kv1.2/2.1 paddle chimera (gray; PDB ID code 2R9R) and RTx/DkTx-bound TRPV1 (cyan; PDB ID code 5IRX) using TM align (28). Backbone rmsd = 2.5 Å and TM score = 0.75. (B) Structure-based sequence alignment of Shaker (black), TRPV1 (red), and the C11 pore chimera in the transmembrane region spanning from S4 through S6 with conserved residues shown in bold. (C) Representative time courses for recording membrane currents at −60 and +60 mV from an uninjected oocyte (Left; control) and an oocyte injected with the C11 chimera (Right). Cells were held at −60 mV and voltage was stepped to +60 mV for 100 ms at 3-s intervals. Mean inward current at −60 mV (Lower symbols) and outward current at +60 mV (Upper symbols) are plotted vs. time. RTx (100 nM), K2K2 (5 µM), AgTx-2 (100 nM), and Zn2+ (2 mM) were applied to the extracellular solution as indicated by horizontal bars. Dotted blue lines denote the zero-current level. The external recording solution contained (in millimoles/liter): 100 KCl, 10 Hepes, 2 MgCl2, pH7.4. (D) Representative current traces from control (Top) and C11 chimera (Bottom) before (Left) and after (Right) the addition of 5 µM K2K2 (Vhold = −60 mV; test depolarizations from −60 to +60 mV in 10-mV increments). (E) I–V relations for membrane currents recording in the presence of 5 µM K2K2 in control uninjected oocytes (black symbols) and C11 injected oocytes (red symbols) obtained from experiments as in D (mean ± SEM for n = 4). (F) Normalized I–V relations before (black symbols) and after K2K2 application (5 µM, red symbols), followed by application of AgTx-2 (100 nM, green symbols) and then Zn2+(2 mM, blue symbols), obtained from experiments as in D. Currents were normalized to the amplitude of current in the presence of 5 µM K2K2 at +60 mV (mean ± SEM for n = 7).