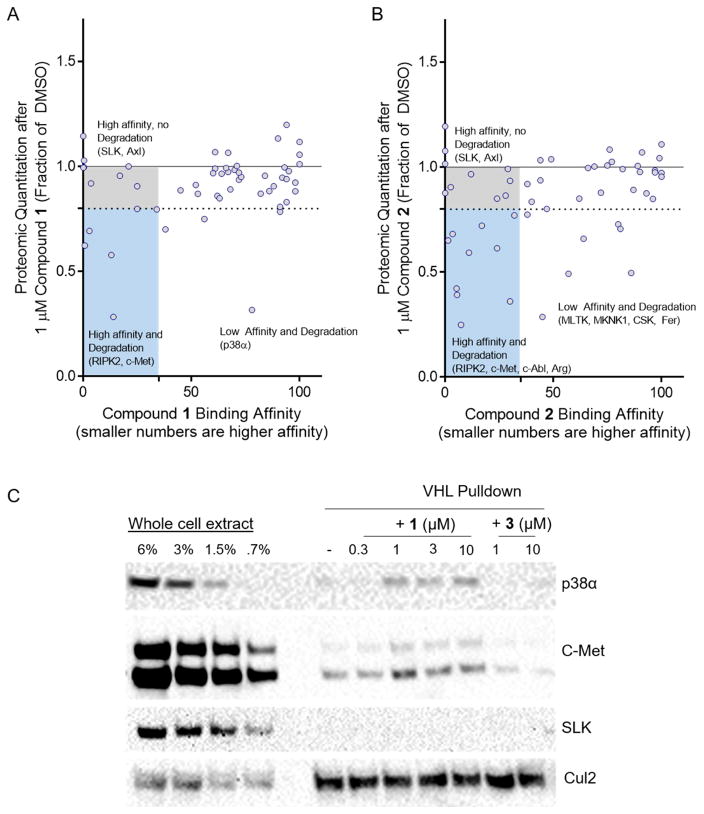
Figure 3. Affinity does not correlate with degradation efficiency, and stable ternary complexes more robustly predict degradation.
(A) Comparison of VHL PROTAC 1’s degradation efficiency and binding affinity for 54 kinases. For each foretinib-binding kinase expressed in MDA-MB-231 cells, the fraction remaining by whole cell proteomics after treatment with 1 μM VHL PROTAC 1 is shown on the Y-axis as a fraction of the DMSO-treated samples. PROTAC’s affinity for that target is shown on the X-axis as a percent of control (KinomeScan). A target is considered degraded if it’s percent of DMSO is below 80%, and a target is considered to bind to the PROTAC if it’s percent of control is less than 35%. (B) Same as in (A), except for CRBN PROTAC 2. (C) Degraded kinases form stable ternary complexes with VHL. GST-tagged VHL/Elongin B/Elongin C were immobilized on glutathione beads and incubated with whole cell extract of MDA-MB-231 cells with the indicated concentrations of compounds 1 or 3. The beads were washed and bound proteins eluted with SDS buffer and analyzed by western blot. See Figure S2 for more robust characterization of degradation and affinity, and Figure S3C for further ternary complex blots of degraded targets.