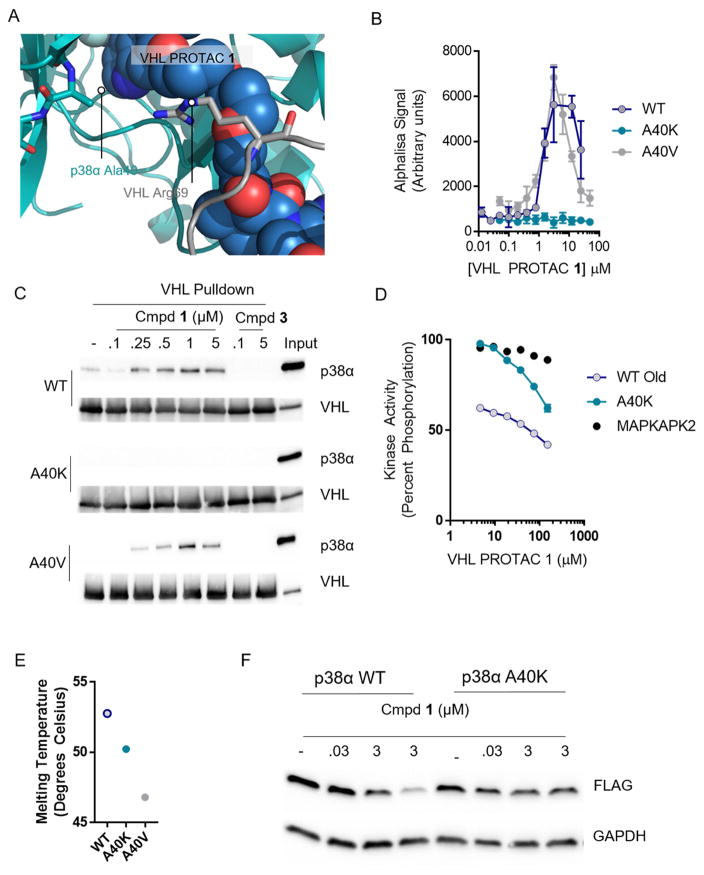
Figure 4. Favorable protein-protein interactions stabilize the compound 1-induced complex between p38α and VHL.
(A) VHL, PROTAC 1, and p38α were docked and a 120 ns molecular dynamics simulation relaxed the structure. Alanine 40 on p38α interacts with the kinked linker region of PROTAC 1. (B) Proximity-based AlphaLisa detects a ternary complex between GST-VHL and His-p38α. VHL PROTAC 1 and purified p38α and VHL were incubated in the presence of Glutathione Donor and Anti-his acceptor beads, and the extent of ternary complex formation was assessed by excitation at 680 nm and detection of emission at 615 nm. (C) Stable interaction between p38α and VHL are interrupted by the A40K mutation. Immobilized VHL/EloB/EloC was used as a bait to trap purified p38α in the presence of the indicated concentrations of compound 1 or 3. The far right lane represents a 1:25 dilution of the total protein used in each pulldown reaction. (D) Despite inhibition of ternary complex formation, the A40K mutation is inhibited by VHL PROTAC 1 to a similar extent as measured by a Z′Lyte cascade assay in which active p38α phosphorylates inactive MAPKAPK2 which phosphorylates a FRET-pair-containing peptide. Active MAPKAPK2 (without p38α protein) was also included as s control. (E) Thermal Stability of the WT, A40K, and A40 mutants are similar, as assessed by Sypro Orange fluorescence with increasing temperature. (F) Overexpressed wildtype p38α is degraded in cells, but not the A40K mutant. HeLa cells were transfected with a pcDNA5 vector containing FLAG-tagged p38α (wildtype or p38α), and then treated with VHL PROTAC 1 at the indicated concentrations for 24 hours.