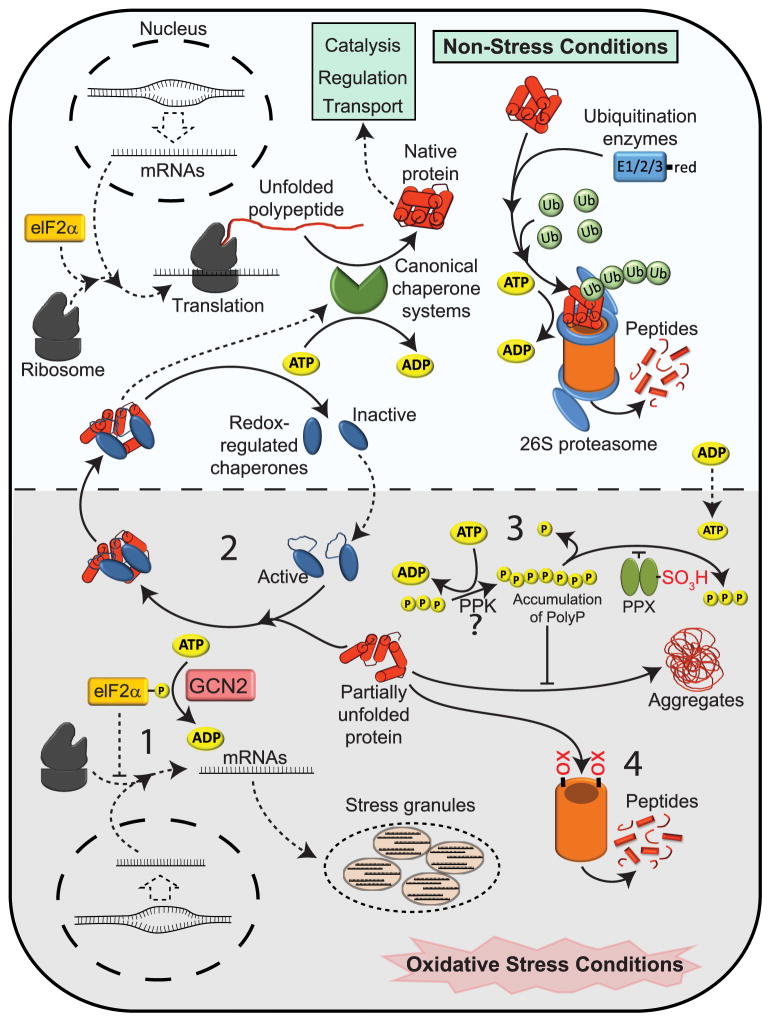
Figure 1. Protein Homeostasis During Oxidative Stress - A Multilayer Approach.
Under non-stress conditions, protein folding occurs both co-and posttranslationally and is supported by ATP-dependent canonical chaperones. Once natively folded, the proteins fulfill their expected functions until degradation signals trigger ubiquitination and subsequent degradation by the ATP-dependent 26S proteasome. Redox-regulated chaperones are inactive but might exert non-stress related enzymatic functions. Under oxidative stress conditions, cellular ATP levels drop, reducing the activity of ATP-dependent processes. To protect cells against the accumulation of irreversible damaged protein aggregates, a number of ROS and/or RCS-mediated changes occur that either directly or indirectly contribute to 1) reduction of new protein synthesis; 2) activation of ATP-independent chaperones; 3) conversion of ATP into polyphosphate, an effective inorganic chaperone; and 4) increase in ATP-independent 20S proteasome activity. Please see text for more details.