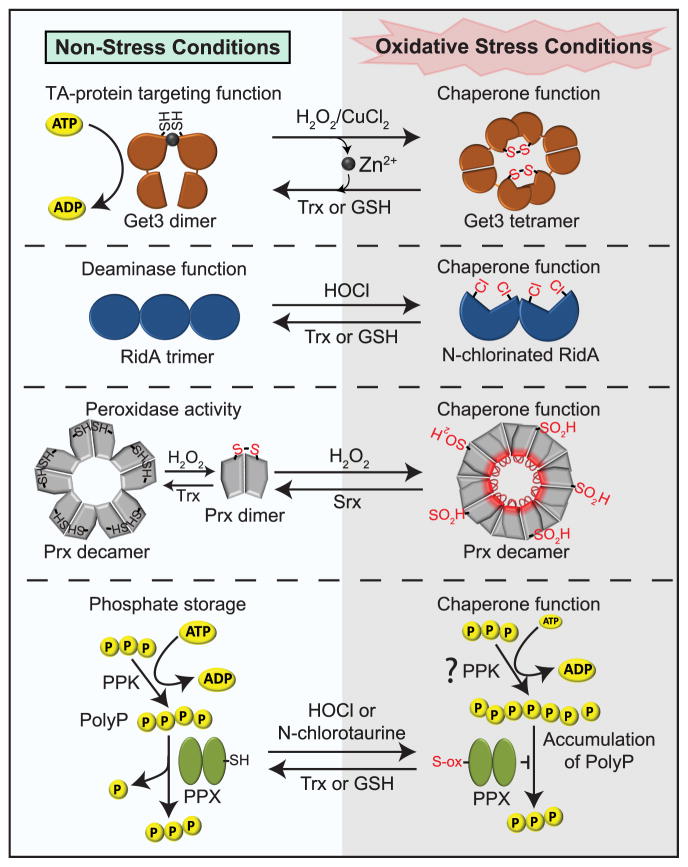
Figure 2. Decreasing Protein Aggregation by Posttranslationally Increasing the Chaperone Pool.
Many of the proteins that serve as chaperones under oxidative stress are dual-function proteins, with distinct enzymatic functions under non-stress conditions. Under oxidative stress conditions, Get3, RidA and peroxiredoxin undergo reversible oxidative modifications that abolish their enzymatic functions and turn them into highly effective ATP-independent chaperones. In addition, bacteria convert ATP into long chains of polyphosphate (polyP), which serve as highly effective chemical chaperones. The transient accumulation of polyP appears to be triggered by a factor that stimulates polyP synthesis during oxidative stress, and mediated by the oxidative inactivation of polyphosphatase PPX, the enzyme that degrades polyP. Please see text for more details.