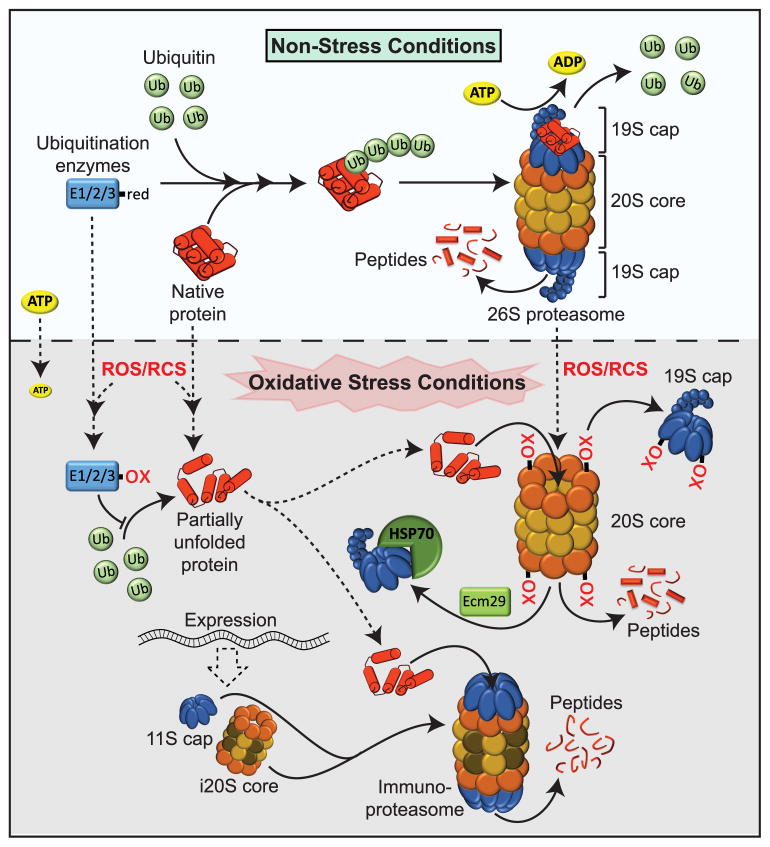
Figure 3. Protein Degradation During Oxidative Stress.
Under non-stress conditions, the majority of proteins targeted for destruction are ubiquitinated and degraded by the 26S proteasome, a hollow barrel-shaped 20S core particle that is capped by two 19S (blue) ATP-dependent regulatory particles. Two outer α-rings (orange) tightly control the gate opening and two inner β-rings (yellow) harbor the proteolytic activity. Proteins of the 19S cap recognize the ubiquitinated client proteins, remove the ubiquitin moiety and mediate the opening of the α-ring gate structure. They furthermore provide the energy to unfold and feed the polypeptide chain into the interior of the barrel-like 20S structure for proteolysis. Under oxidative stress conditions (gray shaded area), partially unfolded proteins directly interact with the α-rings of the 20S proteasome through hydrophobic interactions, eliminating the need for ATP as well as 19S cap or ubiquitination enzymes, both of which contain redox sensitive cysteines. Thiol oxidation of proteins in the α-ring promote further opening of the 20S gate structure, and support disassembly of the 19S caps, which are sequestered by Ecm29 and Hsp70. Chronic oxidative stress induces the expression of an alternative regulatory 11S cap and three alternative β—subunits (brown), which assemble into the 20S immunoproteasome (i20S). Proteolytoc digest by the i20s core generates distinct peptides. The 11S caps binds to both existing 20S and newly assembled i20S proteasomes, functions ATP-independently and increases the protein turnover of the proteasome.