Figure 2. Control of hepatic glycogen metabolism.
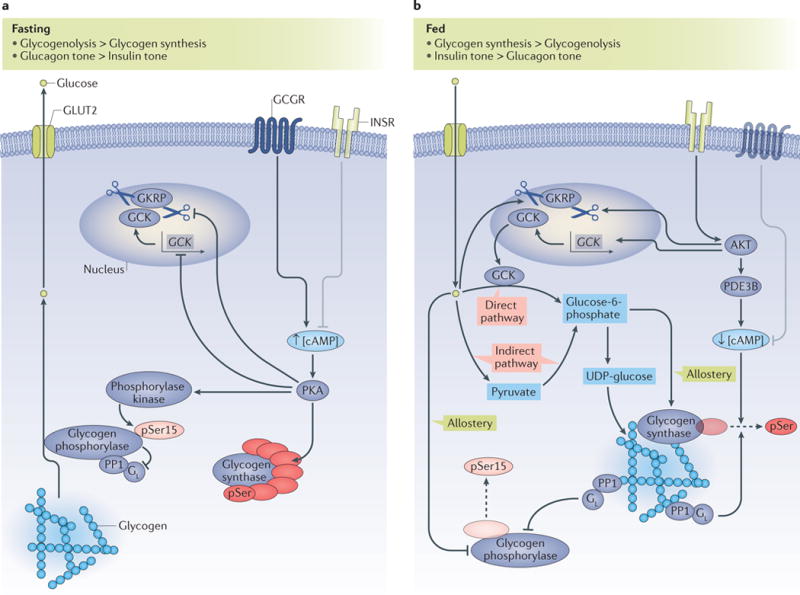
a| Under fasted conditions, glycogenolysis is activated and glycogen synthesis is suppressed. Activation of the glucagon receptor (GCGR) induces increased intracellular concentrations of cyclic AMP (cAMP; indicated by an up arrow), which leads to the activation of protein kinase A (PKA). Activated PKA inhibits the transcription of the glucokinase (GCK) gene; inhibits the dissociation of GCK from glucokinase regulatory protein (GKRP), and thus induces the nuclear sequestration of GCK; phosphorylates, and thus inactivates, glycogen synthase; and phosphorylates and activates phosphorylase kinase, which activates glycogen phosphorylase by phosphorylating Ser15. Phosphorylated, active glycogen phosphorylase also binds to and inhibits the GL subunit of protein phosphatase 1 (PP1), which prevents the PP1-dependent dephosphorylation and inactivation of glycogen synthase. The coordinated activation of glycogen phosphorylase and inhibition of glycogen synthase result in net glycogenolysis. b | Under fed conditions, hormonal and allosteric mechanisms coordinate the stimulation of glycogen synthesis through direct and indirect pathways. Glucose promotes the dissociation of GCK from GKRP, which leads to the cytoplasmic translocation of GCK; glucose also allosterically inhibits glycogen phosphorylase. The ‘direct pathway’ of glycogen synthesis involves the conversion of glucose to glucose-6-phosphate and its subsequent incorporation into glycogen, with all six carbons of the glucosyl unit intact. The ‘indirect pathway’ of glycogen synthesis involves the conversion of glucose to pyruvate, and pyruvate to glucose-6-phosphate, before incorporation into glycogen. Glucose-6-phosphate both allosterically activates glycogen synthase and is a substrate for glycogen synthesis. Insulin activates AKT, which, in turn, induces the transcription of GCK and the cytoplasmic translocation of GCK and activates phosphodiesterase 3B (PDE3B), which decreases intracellular levels of cAMP (indicated by a down arrow) and leads to the inhibition of the PKA-dependent processes described in part a. Active PP1 with its GL targeting subunit dephosphorylates and inactivates glycogen phosphorylase, and dephosphorylates and activates glycogen synthase. The coordinated inhibition of glycogen phosphorylase and activation of glycogen synthase result in net hepatic glycogen synthesis. In parts a, b the insulin receptor (INSR) and glucagon receptor (GCGR) are shown in faded colours for context, and grey inhibitory arrows depict the processes in which they are not dominant. The dashed arrows indicate dephosphorylation. Small up and down arrows indicate an increase or decrease, respectively, in protein level or activity. GLUT2, glucose transporter 2; pSer15, phosphorylated Ser15.
