Figure 3. Framework for understanding the insulin-dependent regulation of hepatic glucose metabolism.
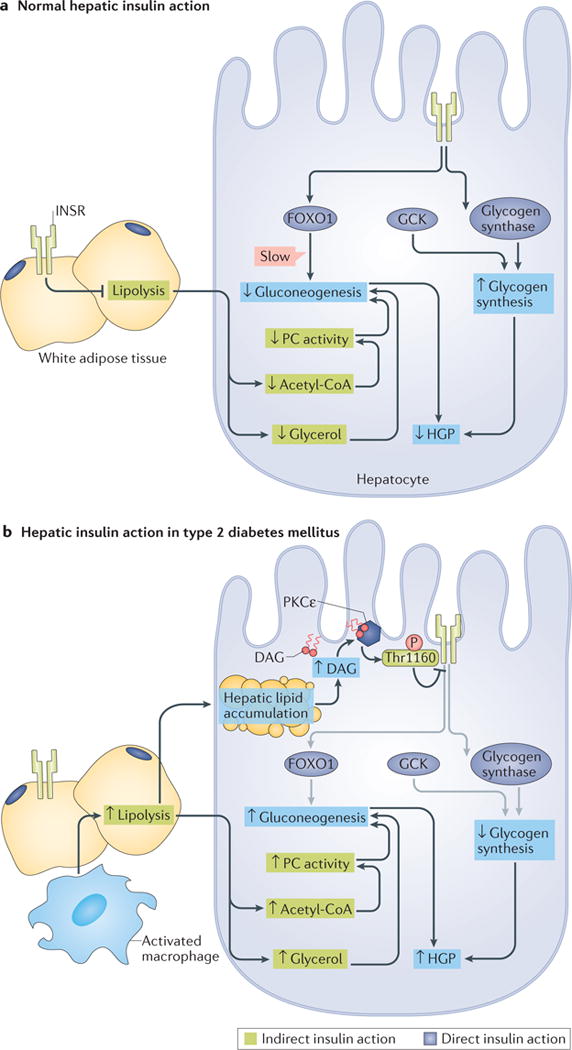
a| Under normal physiological conditions, the direct actions of hepatocellular insulin (dark blue boxes) primarily facilitate net hepatic glycogen synthesis through the activation of glucokinase (GCK) and glycogen synthase. Insulin receptor (INSR)-dependent inactivation of Forkhead box O1 (FOXO1) decreases the transcription of gluconeogenic genes, but this is a relatively slow mechanism that does not mediate the acute suppression of hepatic gluconeogenesis by insulin. Suppression of lipolysis in white adipose tissue (WAT), which is an indirect mechanism of hepatic insulin action (green boxes), acutely suppresses hepatic gluconeogenesis by decreasing the delivery of nonesterified fatty acids (NEFA) and glycerol to the liver, which results in reduced acetyl-CoA-dependent activation of pyruvate carboxylase (PC), and decreased gluconeogenesis from glycerol. The direct stimulation of net hepatic glycogen synthesis and the indirect suppression of hepatic gluconeogenesis collectively suppress hepatic glucose production (HGP). b | In type 2 diabetes mellitus (T2DM), the direct (dark blue boxes) and indirect (green boxes) effects of insulin are impaired through different mechanisms. Lipid-induced hepatic insulin resistance increases hepatic levels of diacylglycerol (DAG), which results in the activation of protein kinase Cε (PKCε) and thereby impairs direct hepatic insulin signalling through PKCε-dependent phosphorylation of INSR at Thr1160. This inhibits the INSR-dependent stimulation of hepatic glycogen synthesis in response to insulin. Impaired INSR signalling is indicated by grey arrows. In WAT, macrophage activation and consequent inflammatory signalling, as well as intrinsic adipocyte dysfunction, increase lipolysis and promote adipocyte insulin resistance, resulting in the continued delivery of NEFA and glycerol to the liver despite high plasma concentrations of insulin. Continued delivery of NEFA and glycerol to the liver promotes hepatic lipid accumulation and PKCε-mediated hepatic insulin resistance, and promotes gluconeogenesis by increasing hepatic acetyl-CoA and gluconeogenesis from glycerol. Impaired net hepatic glycogen synthesis and unrestrained hepatic gluconeogenesis together lead to increases in HGP. Small up and down arrows indicate an increase or decrease, respectively, in protein level or activity. DAG, diacylglycerol.
