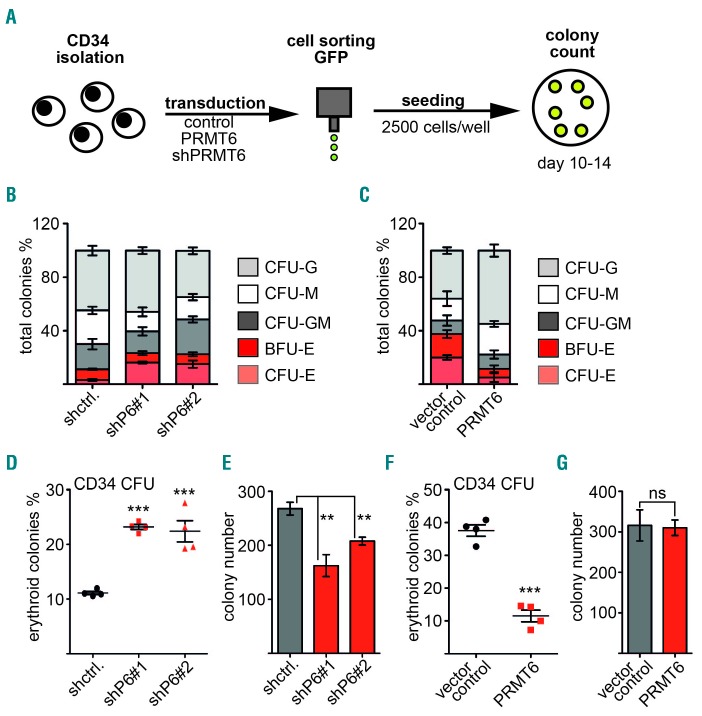
Figure 1.
PRMT6 inhibits erythroid differentiation. (A) Schematic workflow of the colony-forming unit (CFU) assay. Human CD34+ cells were transduced with PRMT6 knockdown (shPRMT6), PRMT6 expression, or control vector. Transduced GFP+ cells were sorted by FACS and subjected to the CFU assay. Colonies were counted on day 10–14 after seeding. (B, C) CFU assay of CD34+ cells upon PRMT6 knockdown and overexpression. Human CD34+ cells were transduced with PRMT6 knockdown vector (shPRMT6), PRMT6 expression vector, or control vector. Transduced GFP+ cells were sorted by FACS and subjected to a CFU assay. Colonies were counted 10–14 days after seeding. (B) CFU assay upon knockdown of PRMT6 using two different shRNA. Unspecific shRNA was used as a control. (C) CFU assay upon PRMT6 overexpression. Empty vector serves as the control. CFU-G colony-forming unit-granulocyte, CFU-M colony-forming unit-monocyte, CFU-GM colony-forming unit-granulocyte, monocyte, BFU-E burst forming unit-erythroid, CFU-E colony forming unit-erythroid. (D) Knockdown of PRMT6 using two different shRNA (shP6) enhances erythroid differentiation of CD34+ cells in the CFU assay. (E) The total number of colonies in the CFU assay after PRMT6 knockdown is shown. (F) The relative frequency of erythroid colonies (in percent) was decreased upon PRMT6 overexpression compared to the control. (G) The total number of colonies in the CFU assay after PRMT6 overexpression is shown. Error bars show the standard deviation calculated from at least four determinations. The P-values were calculated using the Student t-test. *P<0.05; **P< 0.01; ***P<0.001.