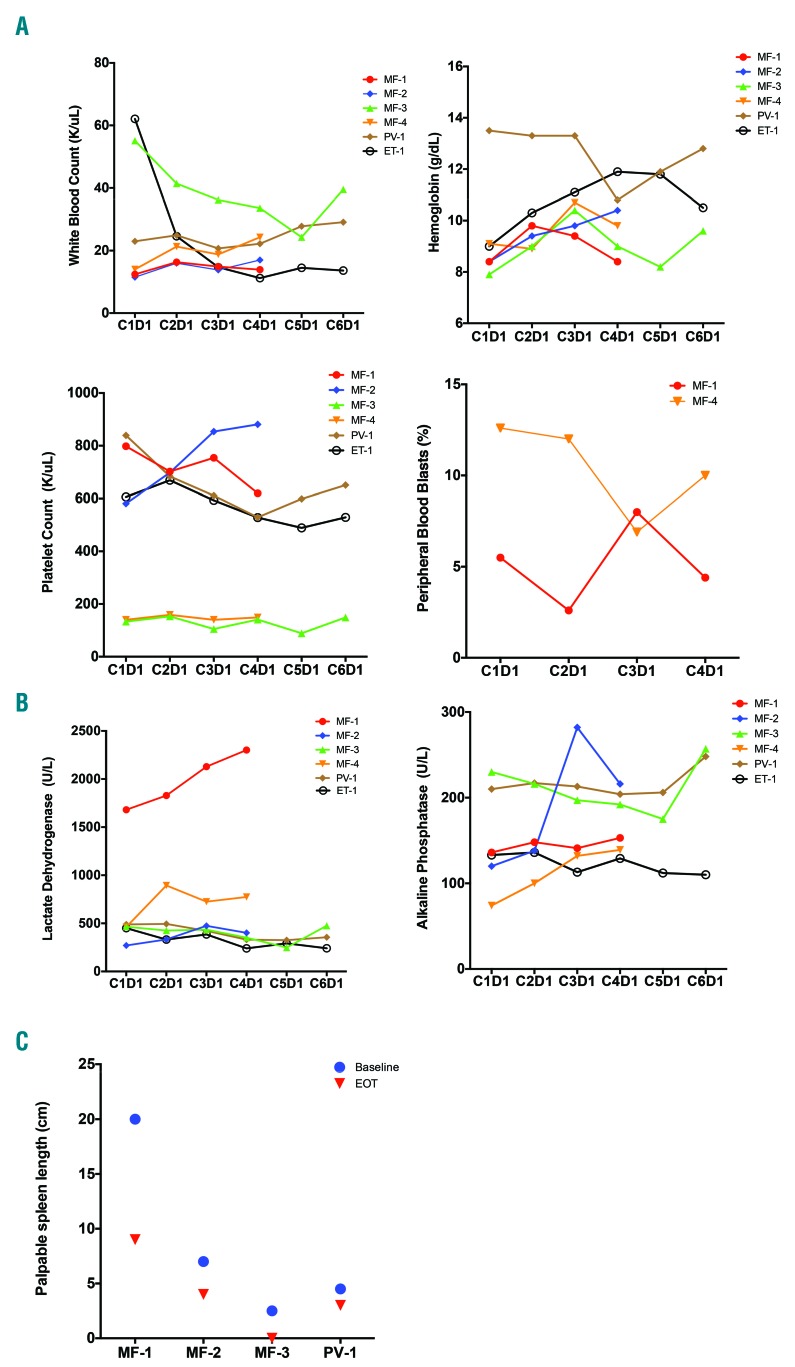
Figure 1.
Impact of AUY922 treatment on clinical parameters. (A) Peripheral blood counts for treated subjects in cycles 1–6 or until time of withdrawal from study if before cycle 6, including white blood count, hemoglobin, platelet count and peripheral blood blast count. (B) Lactate dehydrogenase and alkaline phosphatase levels in cycles 1–6. (C) Measurement of spleen length by palpation in the midclavicular line below the left costal margin at time of baseline and end of treatment (EOT) assessments.