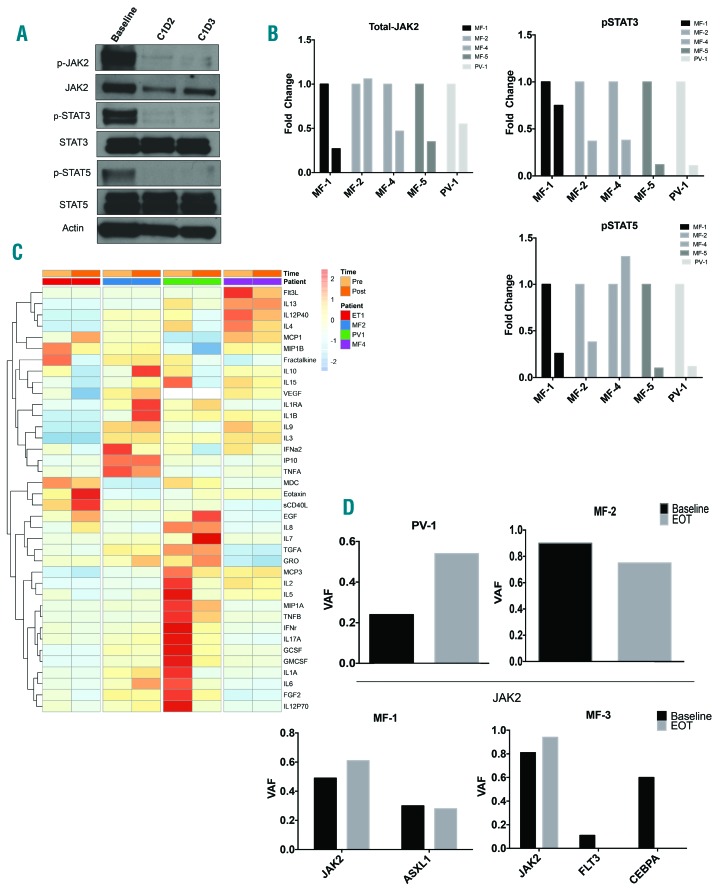
Figure 2.
Impact of AUY922 treatment on pharmacodynamic parameters. (A) Representative Western blot analysis of peripheral blood granulocyte lysate at baseline (prior to drug infusions), one day post infusion (C1D2) and two days post infusion (C1D3) of patient MF-5. (B) Densitometric analysis of Western blots performed on study patients at baseline and one day post infusions (C1D2). Bar graphs display fold change compared to baseline. (C) Peripheral blood cytokine analysis performed at baseline (pre) and cycle 4 day 1 (post) infusion. Heat map depicts row normalized z-scores of cytokine abundance. (D) Variant allele fraction (VAF) based on next-generation sequencing performed on peripheral blood granulocytes at baseline and end of treatment (EOT). Top row represents patients with only JAK2V617F mutation detected (PV-1, MF-2). Bottom row represents patients with more than one mutation detected (MF-1, MF-3). PV: polycythemia vera; MF: myelofibrosis.