Abstract
Corticosteroids such as prednisolone and dexamethasone have been established as up-front therapy for the treatment of newly diagnosed immune thrombocytopenia. Recent studies have indicated that other treatments such as rituximab or thrombopoietin receptor agonist can also be effective choices. We performed a systematic review and network meta-analysis to establish a clinically meaningful hierarchy of efficacy and safety of treatments for newly diagnosed primary immune thrombocytopenia in adults. Randomized controlled trials evaluating medical treatments for newly diagnosed immune thrombocytopenia were included. Reviewers independently extracted data and assessed the risk of bias. The main outcome was the sustained response (platelet count >30×109/L for 3–6 months after completion of treatments), while overall response (platelet count >30×109/L for 2–4 weeks after initiation of the up-front treatment) and therapy-related adverse events were the secondary endpoints. A total of 21 randomized controlled trials (1898 patients) were included in this study. Our main findings were a significantly better sustained response in the recombinant human thrombopoietin+dexamethasone and rituximab+dexamethasone arms compared to those of conventional therapies (prednisolone and dexamethasone monotherapy). Moreover, recombinant human thrombopoietin+dexamethasone and +prednisolone improved early overall response compared to prednisolone, dexamethasone, and rituximab-containing regimens. Therapy-related adverse events showed similar profiles and were tolerable in all treatment arms. Regimens containing recombinant human thrombopoietin agonist may be beneficial up-front therapies in addition to the conventional corticosteroid monotherapies. Future head-to-head trials including these regimens and rituximab-containing treatments are necessary in order to overcome the limitations of the small number in our study and determine the most suitable initial therapies for newly diagnosed immune thrombocytopenia.
Introduction
Newly diagnosed primary immune thrombocytopenia (ITP) is characterized by platelet destruction due to acquired autoantibodies against the platelets and relatively impaired platelet production in the bone marrow,1,2 leading to an increased risk of bleeding.3
The conventional treatments for symptomatic newly diagnosed ITP include corticosteroids; previous guidelines recommended either prednisolone/prednisone (PSL) or high-dose dexamethasone.4,5 In addition, intravenous immune globulins, eradication of Helicobacter pylori, and methylPSL have long been used solely or in combination with other corticosteroids.5 Recently, rituximab in addition to dexamethasone has been shown to be more effective than dexamethasone monotherapy.6 Moreover, thrombopoietin receptor agonists (TPO-RA), including eltrombopag, romiplostim, and recombinant human thrombopoietin (rhTPO), may be reasonable choices given their ability to enhance platelet production in the bone marrow.7
Thus, while there are multiple therapeutic options for up-front treatment of newly diagnosed ITP, there is little evidence to identify the best option for early and sustained recovery of platelet counts without severe adverse events. Several randomized controlled trials (RCT) have compared two specific treatment regimens (e.g., dexamethasone versus PSL and rituximab versus placebo); related systematic reviews and meta-analyses have summarized these results.6,8 However, as there are so many therapeutic options, it is nearly impossible to cover all the combinations in direct head-to-head RCT in order to determine the optimal choice.
Recent developments in the application of network meta-analysis may possibly be helpful to overcome this limitation.9 In contrast to conventional pairwise meta-analyses, this method enables indirect comparisons between treatments used in different trials.10 We, therefore, performed a systematic review and network meta-analysis in order to establish a clinically meaningful hierarchy for the efficacy and safety of newly diagnosed ITP treatments in adults through the integration and synthesis of all available evidence.
Methods
Search strategies and study selection
The search strategies are outlined in Online Supplementary Tables S1 and S2. The patients were limited to adults (16 years or older) with newly diagnosed primary ITP; RCT using other definitions of ITP (such as “acute ITP”) were included only if their definitions matched the “newly diagnosed ITP” defined as ITP of less than 3 months’ duration11 without preceding treatments. Those with secondary thrombocytopenia or those who had had previous therapeutic interventions for ITP were excluded.
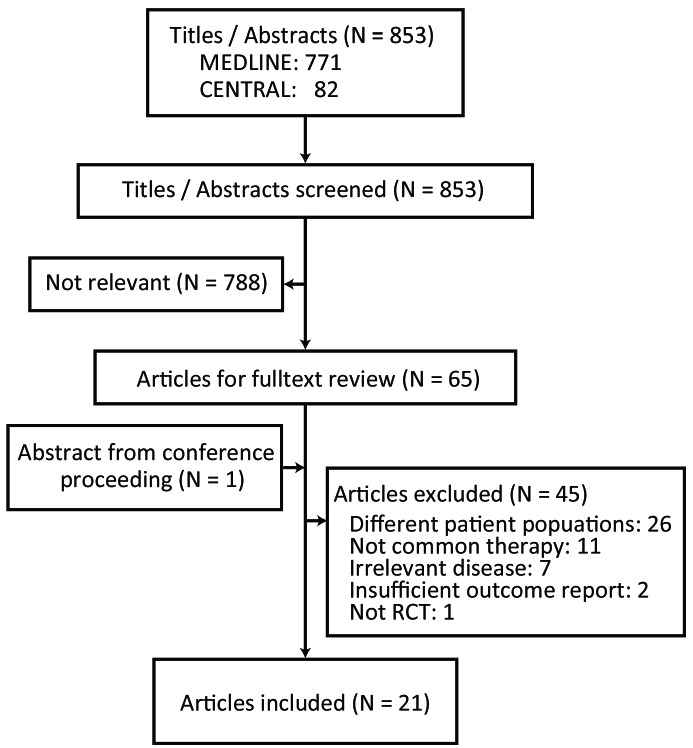
Two review authors scanned the titles and abstracts of the studies identified by the electronic search strategies in order to assess their eligibility. The two authors then independently evaluated the full-text versions of each potentially relevant study for inclusion in the meta-analysis. Disagreements between authors were resolved by discussion. If necessary, arbitration was provided by the senior authors. When missing information inhibited the evaluation of a study, further information was sought from the original authors or other possible sources. The study selection process is reported in a PRISMA flow diagram (Figure 1).
Figure 1.
Study schema. Flow diagram showing the process of identifying and selecting relevant studies.
Data extraction and quality assessment
Data from the included trials were independently extracted by two review authors using a structured, pilot-tested, data extraction form (Online Supplementary Table S3). Differences in data extraction were resolved either by discussion or by consultation with the senior authors.
These review authors also independently assessed the eligible studies for bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions. We evaluated the risk of bias as low, high, or unclear using an assessment form designed for the topic of this review (Online Supplementary Table S4). Any disagreements were discussed with the senior authors until a consensus was obtained.
Data synthesis and analysis
We performed a random effects network meta-analysis using STATA13 software (StataCorp, College Station, TX, USA). Evidence from both direct (head-to-head trials) and indirect (using common comparators without actual head-to-head trials) comparisons was combined in the analysis. The primary outcome was the incidence of long-term sustained response (SR; platelet counts >30×109/L for 3 – 6 months after the completion of treatments). The secondary endpoints included the incidence of (i) early overall response (OR; platelet count >30×109/L in 2 – 4 weeks after the initiation of the up-front therapy) and (ii) severe adverse events [grade 3 or more according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0]. All of our treatment effects were measured as dichotomous data, and were presented as the summaries of the risk ratios (RR) with 95% confidence intervals (CI). A surface under the cumulative ranking curve (SUCRA) was also used to provide a hierarchy of the efficacy and the risk of adverse events of the treatments,12 where SUCRA values of 100% indicated the most effective treatment or the treatment with the least risk of adverse events, and values of 0% indicated the least effective and the highest risk treatment.
Results
Identification of studies
The study identification and selection process is illustrated in Figure 1. From the primary search, 11 studies were excluded because of rare and uncommon therapeutic interventions; these studies dealt with gamma globulins in specific solvent,13,14 a proton pump inhibitor (monotherapy to eradicate Helicobacter pylori),15 vinblastine,16 deflazacort,17 and various Chinese herbal medicines.18–21 Two RCT dealing with intravenous anti-D globulin were excluded from data integration, because one (comparing anti-D versus routine care) did not report either early OR or long-term SR,22 and the other compared low- and high-dose anti-D without reference to other treatment arms,23 resulting in a node unconnected to the other treatment network. A search of conference proceedings revealed one additional relevant study (abstract only). Thus, in total, we included 21 RCT that involved 1898 adult patients with newly diagnosed primary ITP. Individual patients’ data were not available in any study.
Study characteristics
The studies were conducted worldwide including North America, Europe, Africa, and Asia, and were published between 1985 and 2016 (Table 1).24–44 The median age of the participants ranged from 25 to 54 years. Only patients with primary newly diagnosed ITP were included, and RCT dealing with secondary ITP were excluded. Inclusion criteria in each RCT for the initial platelet count or actual platelet count were reported in 19 RCT, with most having a platelet count <30×109/L as an inclusion criterion (Table 1). The interventions were initiated rapidly after the diagnosis (generally within 2 weeks), and they consisted mainly of PSL, dexamethasone, rituximab, and their combinations; while intravenous immunoglobulin, eradication of Helicobacter pylori (using amoxicillin, clarithromycin, and rabeprazole), methylprednisolone, and rhTPO were also used. Rituximab was administered weekly at the dose of either 375 mg/m22,37 or 100 mg/body33,38,39 as combination therapy with dexamethasone. Low-dose (100 mg/body) rituximab was selected mainly because of concerns regarding infectious risk33. All three RCT dealing with rhTPO used TPIAO® manufactured by 3SBio Inc. in China, and rhTPO was administered for 1 – 2 weeks. As for the PSL arm, four studies29,30,35,38 used prednisolone, while prednisone was used in nine studies;24–27,32,34,40,42,44 these data were combined in our meta-analysis. PSL was categorized as low dose if the daily dosage was less than 1.0 mg/kg. Among these studies, 16 RCT (1583 patients) covering nine types of interventions reported results on the primary endpoint (long-term SR; Figure 2A).25–27,30–35,37–40,42–44
Table 1.
Summary of included studies.
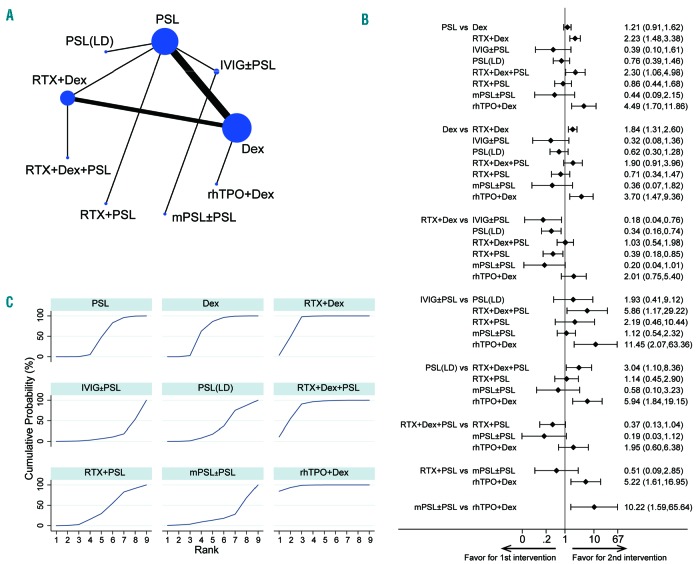
Figure 2.
Results of the network of long-term sustained response comparison. (A) The network of comparisons included in the network meta-analysis for long-term sustained response (SR; platelet counts > 30×109/L at 3 – 6 months). The circle size is proportional to the total number of patients in the treatment group. The line width is proportional to the number of trials comparing the treatment groups. (B) The summary effect estimate (risk ratio of SR) for each combination of treatments. Risk rations are indicated by dots, and 95% confidence intervals by bars. (C) The surface under the cumulative ranking curve (SUCRA) is shown for each treatment.
Risk of bias in the included studies
The risk of bias is graphically summarized in Online Supplementary Figure S1. Information on random sequence generation and allocation concealment was not sufficiently described in many studies (classified as “unclear”). Most of the studies lacked sufficient blinding of participants and personnel, and assessors (classified as “high risk”). These data indicate that some RCT, especially those performed before 2010’s, were performed under poorly designed protocols. Some of the articles were written in Chinese28,32,36,38,39,41,43 and English version were not available; one of the authors (YA) was able to understand the Chinese language, and we also asked the translation service to confirm the detailed meanings. The translated versions of these papers were analyzed by multiple authors just as we did for the other papers written in English. The risk analyses and outcome measure assessment in these Chinese papers were, therefore, properly performed just as for other studies.
Consistency between direct and indirect evidence
Loop-specific tests revealed no significant inconsistency in one available loop (formed by PSL, dexamethasone, and rituximab + dexamethasone arms) within the data network for long-term SR. Based on a design-by-treatment interaction model,45 no significant inconsistency between direct and indirect evidence was identified within the evidence network as a whole (P=0.18). These data support the consistency model in the following analyses.
Outcomes
Long-term sustained response
We analyzed the SR (platelet count >30×109/L11) at 3 – 6 months after the completion of therapies as a dichotomous outcome. The total numbers of patients and the numbers of those who obtained a SR along with its definition in each RCT are displayed in Online Supplementary Table S5. The pooled results demonstrated that (i) rhTPO + dexamethasone and rituximab + dexamethasone produced significantly better responses than dexamethasone or PSL, and (ii) rhTPO + dexamethasone was significantly superior to rituximab + PSL (RR, 5.22; 95% CI: 1.61 – 16.9; P< 0.01) and the RR was also higher when compared to that of rituximab + dexamethasone, though without significance (RR, 2.01; 95% CI: 0.75 – 5.40; P=0.16) (Figure 2B). Here, in the rhTPO + dexamethasone arm, the rhTPO was given for 14 days followed by 4 days of dexamethasone;43 even with such a short therapeutic period, the SR at 3 months after the completion of this up-front treatment marked the fairly good outcome (76.7%) (Online Supplementary Table S5). rhTPO + PSL was not included in this network, and rituximab + PSL did not show any significant difference in comparison with PSL and dexamethasone. The dosage of rituximab (100 mg/body versus 375 mg/m2) was not related to a significant change in long-term SR (RR, 1.10; 95% CI: 0.51 – 2.34; P=0.81).
SUCRA rankings for long-term SR were also evaluated (Figure 2C and Table 2); rhTPO + dexamethasone (97.1%), rituximab + dexamethasone (81.3%), and rituximab + dexamethasone + PSL (81.1%) showed the highest values, followed by dexamethasone (56.2%), PSL (41.5%), and rituximab + PSL (34.6%), while low-dose PSL (27.7%), methylprednisolone (18.5%), and intravenous immune globulins (11.9%) had the lowest values in this ranking.
Table 2.
Ranking of each arm according to the SUCRA values of sustained response and overall response.
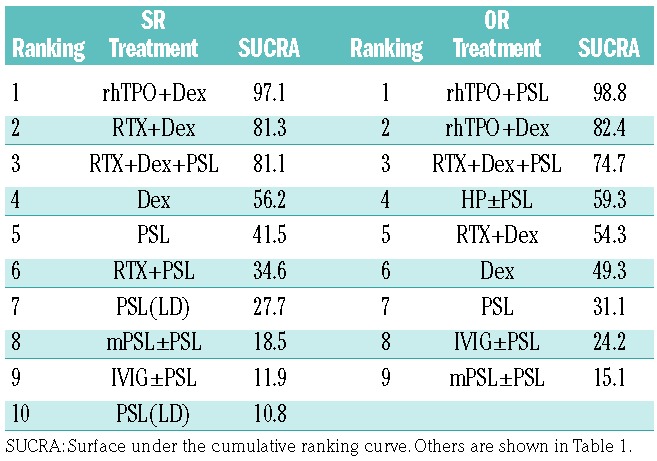
These data indicate that the highest probability of achieving a long-term SR is when ITP is treated with regimens including rhTPO and rituximab in combination with dexamethasone. However, attention should be paid to the small size of the rhTPO + dexamethasone arm (1 study, n=30) when drawing conclusions from these findings (Online Supplementary Table S5).
Early overall response
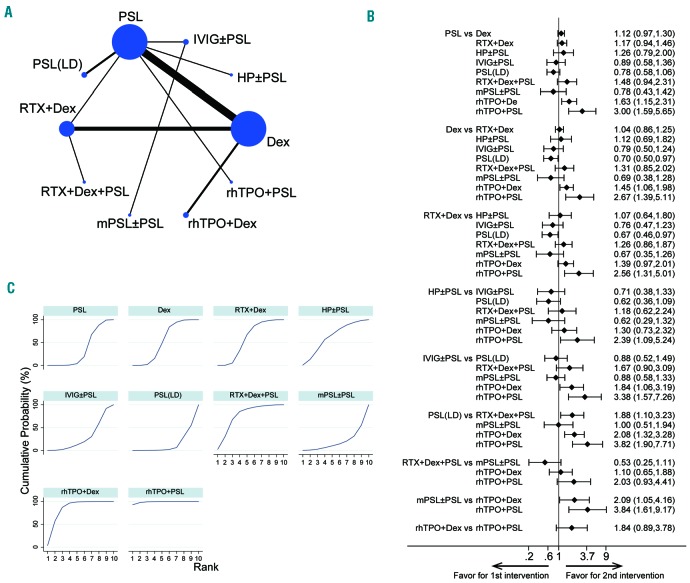
Next, we compared the early OR (platelet count >30×109/L within 2 – 4 weeks after the initial therapies11). Data regarding the incidence of early OR were extracted from 20 studies with 1838 patients;24–33,35–44 the network maps are shown in Figure 3A. The total numbers of patients and the numbers of those who obtained an OR along with its definition in each RCT are presented in Online Supplementary Table S6. The pooled results indicate that the rhTPO arm (both rhTPO + dexamethasone and rhTPO + PSL) produced significantly superior responses compared to the PSL or dexamethasone monotherapy arms (Figure 3B). These two rhTPO regimens also offered better responses than the rituximab + dexamethasone regimen (RR, 1.39; 95% CI: 0.97 – 2.01; P=0.07 for rhTPO + dexamethasone, and RR, 2.56; 95% CI: 1.31 – 5.01; P<0.01 for rhTPO + PSL). The efficacy of rituximab + dexamethasone was almost the same as those of dexamethasone and PSL monotherapies (RR, 1.04: 95% CI: 0.86 – 1.25; P=0.69 for dexamethasone, and RR, 1.17; 95% CI 0.94 – 1.46; P=0.16 for PSL). The dosage of rituximab (100 mg/body versus 375 mg/m2) was not related to a significant change in early OR (RR, 1.13; 95% CI: 0.76 – 1..68; P=0.55).
Figure 3.
Results of the network of long-term sustained response comparison. (A) The network of comparisons included in the network meta-analysis for long-term sustained response (SR; platelet counts > 30×109/L at 3 – 6 months). The circle size is proportional to the total number of patients in the treatment group. The line width is proportional to the number of trials comparing the treatment groups. (B) The summary effect estimate (risk ratio of SR) for each combination of treatments. Risk ratios are indicated by dots, and 95% confidence intervals by bars. (C) The surface under the cumulative ranking curve (SUCRA) is shown for each treatment.
According to the SUCRA values of early OR (Figure 3C and Table 2), rhTPO + PSL (98.8%) had the highest value followed by rhTPO + dexamethasone (82.4%). Rituximab + dexamethasone + PSL (74.7%), H. pylori eradication (only in H. pylori-positive patients; 59.3%), rituximab + dexamethasone (54.2%), dexamethasone (49.3%), and PSL (31.1%) had moderate values, while intravenous immune globulins (24.2%), methylprednisolone (15.1%), and low-dose PSL (10.8%) showed the lowest SUCRA values.
These data indicate that regimens combining rhTPO and corticosteroids (rhTPO + dexamethasone or rhTPO + PSL) may be the optimal choice for obtaining an early OR in newly diagnosed ITP. The small size of the rhTPO + PSL arm (1 study, n=31 patients) and the rhTPO + dexamethasone arm (2 studies, n=53 patients) should be considered as a limitation (Online Supplementary Table S6). This tendency was also confirmed even when OR was determined as a platelet count >50×109/L instead of a platelet count >30×109/L, although the number of included studies was small (Online Supplementary Table S6) and the results were not robust enough (data not shown).
Incidence of adverse events
A total of 16 studies including 1325 patients provided data regarding acute- or chronic-phase adverse events related to each intervention.27,29–37,39–44 Severe adverse events (CTCAE grade 3 or more) were divided into non-hemorrhagic and hemorrhagic events, and the numbers of patients who experienced them are shown in detail and compared among seven interventions (Online Supplementary Table S7). As for non-hemorrhagic adverse events, the pooled data showed no significant differences in incidence (Online Supplementary Figure S2A, B). The SUCRA rankings for adverse events revealed the best score for dexamethasone (69.3%; the lowest risk of adverse events), while rituximab + PSL had the lowest score (19.3%; the highest risk) and other interventions were associated with a modest risk (Online Supplementary Figure S2C). In the dexamethasone arm (12 RCT in total), the incidence of adverse events was 4.1% (22/531), compared to 6.0% (2/33) in the rituximab + PSL arm (including only 1 RCT); almost all of the adverse events were manageable. In studies using rhTPO, emergence of anti-thrombopoietin antibodies was not reported.
As for hemorrhagic events, a total of nine events was observed in the PSL (n=4), rituximab + dexamethasone (n=3), and dexamethasone (n=2) arms ranging from petechiae to intracranial hemorrhage (Online Supplementary Table S7). No clear relationship with each arm and the incidence was detected.
Publication bias
Publication bias was assessed using a funnel plot for the network of long-term SR and early OR (Online Supplementary Figure S3). All the included studies were symmetrically distributed around the vertical line, indicating that there was no significant publication bias in this network analysis.
Discussion
This systematic review and network meta-analysis of the efficacy of up-front treatments for newly diagnosed ITP included 21 trials with 1898 randomly assigned participants. Our main findings showed significantly superior SR for the rhTPO + dexamethasone and rituximab + dexamethasone arms compared to those for the conventional therapies (PSL and dexamethasone monotherapy). Moreover, both rhTPO + dexamethasone and rhTPO + PSL also improved early OR compared to PSL, dexamethasone, or rituximab-containing regimens. Therapy-related adverse events showed similar profiles, and were tolerable in all treatment arms.
The superior efficacy of rhTPO + dexamethasone compared to PSL or dexamethasone monotherapy as an initial treatment for newly diagnosed ITP with regards to long-term SR has been firstly shown in this meta-analysis. This methodology demonstrates more formally what some clinicians have suspected, and the result suggests that TPO-RA may possibly be a first choice for the treatment of newly diagnosed ITP in addition to therapy-resistant or chronic ITP, in which the additive effects of TPO-RA have previously been shown in various RCT and systematic reviews.7,46 Our results are partially supported by previous studies that analyzed blood thrombopoietin concentrations; serum thrombopoietin levels in patients with newly diagnosed ITP are within the normal range or only minimally elevated compared to those of healthy people,11,47 although data on serum thrombopoietin levels were not available in any RCT included in our study. Attention should be paid to the fact that in all the studies rhTPO was combined with PSL or dexamethasone; relatively short-term administration of rhTPO alone (2 weeks) may not have resulted in such good responses. Moreover, it should be noted that rhTPO may be better than even rituximab-containing regimens, which have been widely used recently for the treatment of newly diagnosed ITP after several RCT and systematic reviews reported that these latter are associated with higher efficiency and lower relapse risk than corticosteroid monotherapy.6 The results of this network meta-analysis, which allowed comparisons to be made even without head-to-head RCT (rhTPO versus rituximab), support the necessity for RCT directly comparing rhTPO and rituximab in order to validate our analysis and to determine the first-choice regimen that offers the best results in terms of long-term SR. However, it should be noted that rhTPO, but not romiplostim or eltrombopag, was used as the TPO-RA in this study.
In addition to superior SR, the higher incidence of early OR with rhTPO regimens (compared to PSL, dexamethasone, and rituximab) in this study is also a novel finding. Previous studies have shown that rituximab regimens are not expected to accelerate platelet recovery or to improve early OR compared to corticosteroid monotherapy.31,37 However, rhTPO shortens the platelet recovery period in each RCT;36,41,43 as a result, the data synthesized in this network meta-analysis clearly demonstrated the improvement of early OR. This prominent effect of rhTPO + dexamethasone or + PSL can be obtained because each drug has a different mechanism of action; the former enhances platelet production in the bone marrow,48 while the latter increases the rate of apoptosis of autoantibody-producing lymphocytes and down-regulates macrophage activity responsible for platelet phagocytosis.49 This synergistic effect cannot be obtained from any combinations of various immunosuppressing agents (such as rituximab + dexamethasone).
The serious adverse events were manageable in every treatment arm, even those with rhTPO or rituximab. This may be because (i) rituximab and rhTPO are drugs that generally have low incidences of severe adverse effects, and (ii) these additional treatments promote rapid and sustained platelet recovery, leading to shorter treatment periods and smaller corticosteroid dosages; as a result, steroid-derived adverse events, such as hypertension, glucose intolerance, and infection, may be decreased in these treatment arms.
Other treatment strategies, such as methylprednisolone and intravenous immune globulins, did not show any significant additive effects compared to conventional PSL or dexamethasone monotherapies. Among them, intravenous immune globulins induced platelet recovery within days,26,27 indicating its therapeutic advantages for patients requiring rapid platelet recovery due to extremely severe thrombocytopenia and/or high risk of critical hemorrhage. However, this rapid recovery seems to be temporal and was not related to superior OR and SR.
So far, we have described a methodology that reveals new insights into the efficacy and safely of treatments for newly diagnosed ITP, but this study has some limitations. First, only a few RCT were included for some treatment arms; for example, only one RCT was included for the H. pylori eradication,28 rhTPO + PSL,36 and rituximab + dexamethasone followed by consolidation PSL39 treatment arms, although the number of participants in each RCT was relatively large. This limitation generally results in a higher β error (lower power to detect differences) and a statistically unstable model. Sensitivity analysis excluding these three RCT (H. pylori eradication, rhTPO + PSL, and rituximab + dexamethasone + PSL) resulted in the same conclusions as for SR and OR results (data not shown), supporting the reliability of our results. Future RCT, however, are warranted to overcome this limitation. Including patients after splenectomy may be useful for a more comprehensive analysis. Second, meta-analyses of the outcomes were performed only for SR at 3 – 6 months and OR at 2 – 4 weeks. Other outcomes, such as SR at later time-points (1 – 3 years) or normalization of platelet counts after therapy (>100×109/L instead of 30×109/L), should be analyzed. Unfortunately, the numbers of RCT reporting these outcomes were very limited, so we could not carry out meta-analysis. Nevertheless, our outcomes, such as SR at 3 – 6 months or OR at 2 – 4 weeks, are reasonable enough to evaluate the potency of these regimens because both of these outcomes represent sustained and early therapeutic effects in the management of newly diagnosed ITP.31,44 Third, regarding TPO-RA, only studies dealing with rhTPO were included in our analysis, because we were unable to identify RCT comparing other TPO-RA such as romiplostim and eltrombopag. rhTPO is now commercially available only in China, which will limit the geographical generalization of the findings of this study. Sensitivity analysis excluding these studies using rhTPO36,41,43 resulted in the same conclusions regarding the superiority of rituximab combined with corticosteroid compared to the monotherapy (Online Supplementary Table S8). The other type of rhTPO (pegylated, recombinant humsn megakaryocyte growth and development factor; PEG-rHuMGDF) was developed and tested in the 1990s, but the development of this type ended soon because of the emergence of autoantibodies to PEG-rHuMGDF,50 and no RCT involving this drug were found. Moreover, the efficacy of TPO-RA may differ according to the specific agent used.7 Hence, further RCT are necessary to confirming the benefits of TPO-RA as an initial treatment for newly diagnosed ITP. Lastly, insufficiency in adverse event reporting is another limitation. Detailed information on adverse events was obtained only from 16 RCT (76.2% of the total). The protocol to follow up toxicities may be different in each country, especially in the older studies. Judging from the data obtained, adverse events in the clinical course of ITP treatment are generally manageable, and treatment-related deaths were rarely observed in any of the RCT included in this study. These data suggest that any regimens analyzed in this study are feasible as up-front treatments for newly diagnosed ITP.
In summary, this systematic review and network meta-analysis demonstrated that this approach is reasonable for the analysis of ITP, and it indicated the efficacy of rhTPO in newly diagnosed ITP; the results suggest that rhTPO + dexamethasone or + PSL regimens may be up-front therapeutic options along with conventional corticosteroid monotherapy or rituximab. No significant differences in long-term SR or early OR were detected between patients given the two dosages of rituximab (100 mg/body versus 375 mg/m2), which may have some impact on the current usage of rituximab. However, the lower number of included RCT and patients and higher risk of bias in some RCT could be a limitation in our analysis. The high risk of bias for blinding can work in favor of new interventions (rituximab and rhTPO). Thus, future worldwide head-to-head RCT including these regimens (including romiplostim and eltrombopag) and rituximab-containing treatments are essential in order to determine the most suitable initial therapeutic strategies for newly diagnosed ITP in adults.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/1/163
References
- 1.Psaila B, Bussel JB. Immune thrombocytopenic purpura. Hematol Oncol Clin North Am. 2007;21(4):743–759. [DOI] [PubMed] [Google Scholar]
- 2.Stasi R, Evangelista ML, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost. 2008;99(1):4–13. [DOI] [PubMed] [Google Scholar]
- 3.Cohen YC, Djulbegovic B, Shamai-Lubovitz O, Mozes B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11): 1630–1638. [DOI] [PubMed] [Google Scholar]
- 4.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. [DOI] [PubMed] [Google Scholar]
- 5.Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16): 4190–4207. [DOI] [PubMed] [Google Scholar]
- 6.Chugh S, Darvish-Kazem S, Lim W, et al. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2015;2(2):e75–81. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Gao Z, Chen XP, et al. Efficacy and safety of thrombopoietin receptor agonists in patients with primary immune thrombocytopenia: a systematic review and meta-analysis. Sci Rep. 2016;6:39003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mithoowani S, Gregory-Miller K, Goy J, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016;3(10):e489–e496. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691. [DOI] [PubMed] [Google Scholar]
- 11.Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bussel JB, Eldor A, Kelton JG, et al. IGIV-C, a novel intravenous immunoglobulin: evaluation of safety, efficacy, mechanisms of action, and impact on quality of life. Thromb Haemost. 2004;91(4):771–778. [DOI] [PubMed] [Google Scholar]
- 14.Wolf HH, Davies SV, Borte M, et al. Efficacy, tolerability, safety and pharmacokinetics of a nanofiltered intravenous immunoglobulin: studies in patients with immune thrombocytopenic purpura and primary immunodeficiencies. Vox Sang. 2003;84(1):45–53. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsumi Y, Kanamori H, Yamato H, et al. Randomized study of Helicobacter pylori eradication therapy and proton pump inhibitor monotherapy for idiopathic thrombocytopenic purpura. Ann Hematol. 2005;84(12):807–811. [DOI] [PubMed] [Google Scholar]
- 16.Facon T, Caulier MT, Wattel E, Jouet JP, Bauters F, Fenaux P. A randomized trial comparing vinblastine in slow infusion and by bolus i.v. injection in idiopathic thrombocytopenic purpura: a report on 42 patients. Br J Haematol. 1994;86(3):678–680. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari A, Pasqualetti D, Del Bianco P, Gandolfo GM, Chistolini A, Mazzucconi MG. Prednisone versus deflazacort in the treatment of autoimmune thrombocytopenic purpura: evaluation of clinical response and immunological modifications. Haematologica. 1991;76(4):342–345. [PubMed] [Google Scholar]
- 18.Luo YG, Liu YQ, Hu J. [Clinical study on effect of recombinant roasted licorice decoction combined with low-dose glucocorticoids in treating idiopathic thrombocytopenic purpura]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21(7):501–503. [PubMed] [Google Scholar]
- 19.Yu XQ, Chen HM, Sun JH, Luo M, Lu YL. [Therapeutic efficacy of multigly-cosidorum tripterygium combined with rhIL-11 for immune thrombocytopenia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23(5):1400–1403. [DOI] [PubMed] [Google Scholar]
- 20.Zeng FC, Hong F, Yang YQ. [Clinical study of zhinu-I,-II in treating 61 patients with idiopathic thrombocytopenic purpura]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996;16(4):207–209. [PubMed] [Google Scholar]
- 21.Zhou YM, Hu MH, Yang JM, et al. [Clinical study on idiopathic thrombocytopenic purpura treated with Shengxueling Granule]. Zhong Xi Yi Jie He Xue Bao. 2004;2(6):421–425. [DOI] [PubMed] [Google Scholar]
- 22.George JN, Raskob GE, Vesely SK, et al. Initial management of immune thrombocytopenic purpura in adults: a randomized controlled trial comparing intermittent anti-D with routine care. Am J Hematol. 2003;74(3):161–169. [DOI] [PubMed] [Google Scholar]
- 23.Newman GC, Novoa MV, Fodero EM, Lesser ML, Woloski BM, Bussel JB. A dose of 75 microg/kg/d of i.v. anti-D increases the platelet count more rapidly and for a longer period of time than 50 microg/kg/d in adults with immune thrombocytopenic purpura. Br J Haematol. 2001;112(4):1076–1078. [DOI] [PubMed] [Google Scholar]
- 24.Mazzucconi MG, Francesconi M, Fidani P, et al. Treatment of idiopathic thrombocytopenic purpura (ITP): results of a multicentric protocol. Haematologica. 1985;70(4): 329–336. [PubMed] [Google Scholar]
- 25.Bellucci S, Charpak Y, Chastang C, Tobelem G. Low doses v conventional doses of corticoids in immune thrombocytopenic purpura (ITP): results of a randomized clinical trial in 160 children, 223 adults. Blood. 1988;71(4):1165–1169. [PubMed] [Google Scholar]
- 26.Jacobs P, Wood L, Novitzky N. Intravenous gammaglobulin has no advantages over oral corticosteroids as primary therapy for adults with immune thrombocytopenia: a prospective randomized clinical trial. Am J Med. 1994;97(1):55–59. [DOI] [PubMed] [Google Scholar]
- 27.Godeau B, Chevret S, Varet B, et al. Intravenous immunoglobulin or high-dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: a randomised, multicentre trial. Lancet. 2002;359(9300):23–29. [DOI] [PubMed] [Google Scholar]
- 28.Kong R, Qiu HC, Wu PF, Niu XH, Shen WX, Wang Y. [Clinical significance of Helicobacter pylori in pathogenesis of idiopathic thrombocytopenic purpura]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16(5):1222–1226. [PubMed] [Google Scholar]
- 29.Praituan W, Rojnuckarin P. Faster platelet recovery by high-dose dexamethasone compared with standard-dose prednisolone in adult immune thrombocytopenia: a prospective randomized trial. J Thromb Haemost. 2009;7(6):1036–1038. [DOI] [PubMed] [Google Scholar]
- 30.Bae SH, Ryoo H-M, Lee WS, et al. High dose dexamethasone vs. conventional dose prednisolone for adults with immune thrombocytopenia: a prospective multicenter phase III Trial. Blood. 2010;116:3687.21071611 [Google Scholar]
- 31.Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010; 115(14):2755–2762. [DOI] [PubMed] [Google Scholar]
- 32.Cui ZG, Wei Y, Hou M, Zhao HG, Wang HY. [The efficacy and safety of 2 cycles’ high-dose dexamethasone treatment adult primary immune thrombocytopenia]. Zhonghua Nei Ke Za Zhi. 2011;50(5):401–403. [PubMed] [Google Scholar]
- 33.Li Z, Mou W, Lu G, et al. Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 2011;93(1):91–98. [DOI] [PubMed] [Google Scholar]
- 34.Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119(6):1356–1362. [DOI] [PubMed] [Google Scholar]
- 35.Mashhadi MA, Kaykhaei MA, Sepehri Z, Miri-Moghaddam E. Single course of high dose dexamethasone is more effective than conventional prednisolone therapy in the treatment of primary newly diagnosed immune thrombocytopenia. Daru. 2012; 20(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu SY, Zhuang JL, Zou SH, et al. [A clinical comparative study on treatment of severe newly diagnosed immune thrombocytopenia by recombinant human thrombopoietin combined with glucocorticoid]. Zhonghua Xue Ye Xue Za Zhi. 2013;34(10):883–886. [DOI] [PubMed] [Google Scholar]
- 37.Gudbrandsdottir S, Birgens HS, Frederiksen H, et al. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 2013;121(11): 1976–1981. [DOI] [PubMed] [Google Scholar]
- 38.Li ZY, Li DP, Yan ZL, et al. [Effect of different therapeutic regimens on regulatory T cells in patients of primary immune thrombocytopenia]. Zhonghua Xue Ye Xue Za Zhi. 2013;34(6):478–481. [DOI] [PubMed] [Google Scholar]
- 39.Xing WW, Li ZY, Yan ZL, et al. [Efficacy and safety of low-dose rituximab combined with different dosage of glucocorticoids for immune thrombocytopenia]. Zhonghua Xue Ye Xue Za Zhi. 2013;34(5):409–412. [DOI] [PubMed] [Google Scholar]
- 40.Din B, Wang X, Shi Y, Li Y. Long-term effect of high-dose dexamethasone with or without low-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol. 2015;133(1):124–128. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Huang Q, Wang C, Muhebaier, An L, Wang X. [Efficacy and safety of high-dose dexamethasone combined with rhTPO for newly diagnosed adults with severe immune thrombocytopenia]. Zhonghua Xue Ye Xue Za Zhi. 2016;37(2):134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A Randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naive adult patients with immune thrombocytopenia: EIS 2002 Study. Acta Haematol. 2016;136(2):101–107. [DOI] [PubMed] [Google Scholar]
- 43.Sun M, Wang X, Jiang M, et al. [A clinical analysis of treatment with recombinant human thrombopoietin combined with large doses of dexamethasone in primary immune thrombocytopenia]. Zhonghua Nei Ke Za Zhi. 2016;55(3):202–205. [DOI] [PubMed] [Google Scholar]
- 44.Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296–302. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper KL, Fitzgerald P, Dillingham K, Helme K, Akehurst R. Romiplostim and eltrombopag for immune thrombocytopenia: methods for indirect comparison. Int J Technol Assess Health Care. 2012;28(3):249–258. [DOI] [PubMed] [Google Scholar]
- 47.Makar RS, Zhukov OS, Sahud MA, Kuter DJ. Thrombopoietin levels in patients with disorders of platelet production: diagnostic potential and utility in predicting response to TPO receptor agonists. Am J Hematol. 2013;88(12):1041–1044. [DOI] [PubMed] [Google Scholar]
- 48.Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther. 2015;32(10): 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizutani H, Furubayashi T, Imai Y, et al. Mechanisms of corticosteroid action in immune thrombocytopenic purpura (ITP): experimental studies using ITP-prone mice, (NZW × BXSB) F1. Blood. 1992;79(4):942–947. [PubMed] [Google Scholar]
- 50.Kuter DJ. Milestones in understanding platelet production: a historical overview. Br J Haematol. 2014;165(2):248–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.