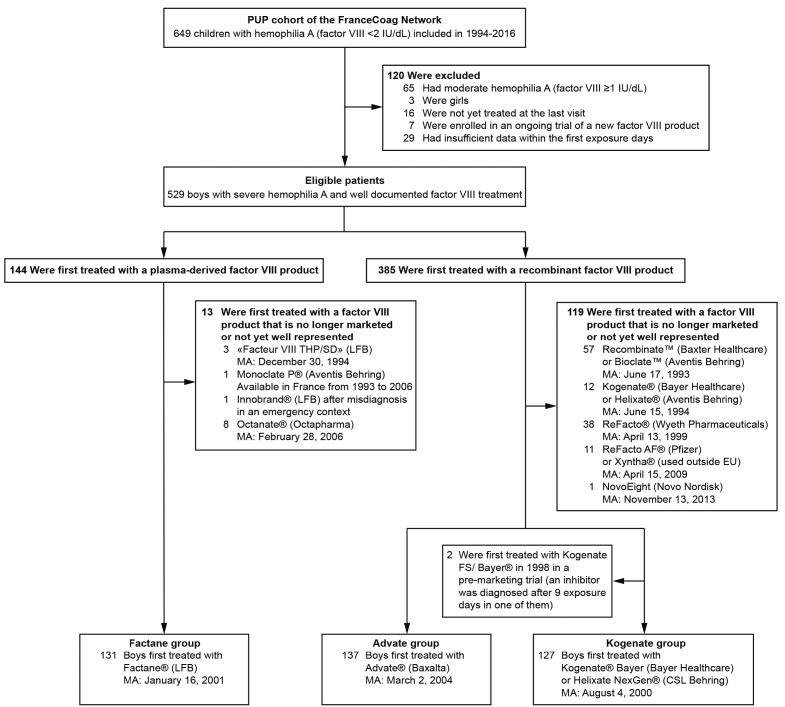
Figure 1.
Patient selection process. At the cutoff date (December 6, 2016), 649 previously untreated patients (PUPs) with hemophilia A (factor VIII <2 IU/dL) had been included in the dedicated cohort of FranceCoag. After the selection process, three groups of boys with severe hemophilia A (factor VIII <1 IU/dL) were formed based on the first factor VIII product received. MA: marketing authorization dates in European Union (or in France for Factane®).