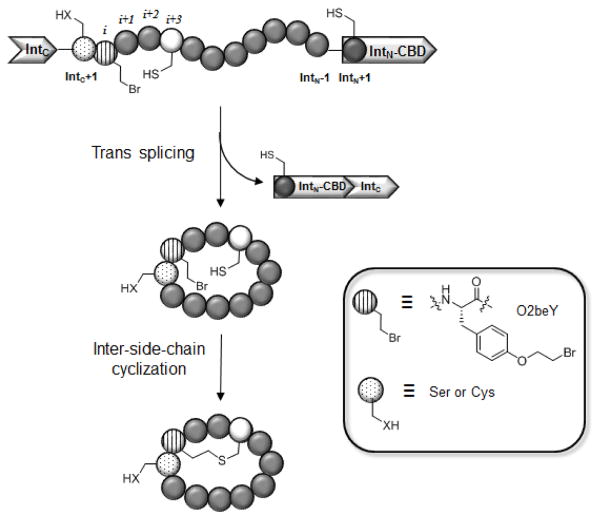
Figure 1.
Overall strategy for the ribosomal synthesis of thioether-bridged bicyclic peptides in E. coli. From the N- to C-terminus, the precursor protein comprises: (a) the C-terminal domain of DnaE split intein (IntC); (b) a variable target peptide sequence; (c) the N-terminal domain of DnaE split intein fused to a chitin binding domain (IntN-CBD). The target peptide sequence contains an initial Ser or Cys residue (dotted circle) at the ‘IntC+1’ site for mediating split intein-catalyzed head-to-tail cyclization and comprises the unnatural amino acid O-(2-bromoethyl)-tyrosine (O2beY, circle with vertical lines) and the reactive cysteine (white circle) for inter-side-chain crosslinking via thioether bond formation.