Summary
Transcription activator-like effectors (TALEs) are important genomic tools with customizable DNA binding motifs for locus-specific modifications. In particular, TALE Nucleases or TALENs have been successfully used in the zebrafish model system to introduce targeted mutations via repair of double stranded breaks (DSBs) either through non-homologous end joining (NHEJ), or by homology-directed repair (HDR) and homology-independent repair in the presence of a donor template. Compared with other customizable nucleases, TALENs offer high binding specificity and fewer sequence constraints in targeting the genome, with comparable mutagenic activity. Here, we describe a detailed in silico design tool for zebrafish genome editing for TALENs and CRISPR/Cas9 custom restriction enzymes using Mojo Hand 2.0 software.
Keywords: TALEN, Customized nucleases, zebrafish, genome editing, Golden Gate, FusX
1. Introduction
TALEs are naturally occurring transcription factors isolated from plant pathogen Xanthomonas (1,2). Each TALE has a DNA recognizing TALE domain made up of a tract of almost identical repetitive units (33–35 amino acid residues) and a partial (or half) repeat unit at the end. Within each unit, the two repeat-variable di-residues (RVDs) are solely responsible for the binding specificity of the unit towards a DNA nucleotide in a highly predictable fashion (3,4). Commonly used RVDs include NI, NN for adenine; HD for cytosine; NK, NN, NH for guanine and NG for thymine (3–6). Because of the 1:1 RVD to nucleotide modularity of the TALE domain, it can be engineered to target almost any DNA sequence in the genome and can be fused with different functional domains including nuclease, transcription activator/repressor, and methyltransferases. TALEs represent important genomic tools for locus-specific modifications (7–14). In particular, TALENs have been extensively used for targeted mutations in vitro and in different model organisms (15–22).
Diverse methodologies have been developed to assemble the modular TALE domain, with the Golden Gate TALEN assembling method (Golden Gate TALEN Kit 2.0) being widely used because of its flexibility, low start-up cost and requirement of minimal, common molecular cloning reagents (23). We previously reported the first use of GoldyTALEN in targeted zebrafish genome editing through both NEHJ and HDR (8). We also described a simple and highly active GoldyTALEN design with only 15 RVDs (or 14.5 TALE repeats) (22). To further facilitate TALEN-mediated high throughput genome editing, we subsequently developed a modified Golden Gate TALEN assembling FusX system (Ma et al., manuscript in preparation). The new system increased assembling efficiency, but shortened assembling time without affecting mutagenic activity and compatibility.
With the rapid development of novel genome engineering tools such as TALENs and CRISPR/Cas9 systems (24), new software tools are needed to aid biologists in designing and constructing high efficiency reagents that can be used to make tailored changes within any model system of interest. Through a better understanding of the cell’s endogenous DNA repair mechanisms, we can improve reagent design and targeting to achieve predictable outcomes. Microhomology mediated end joining (MMEJ) appears to be a dominant repair pathway for TALEN and RNA-guided engineered nucleases (RGEN) induced double stranded breaks and has been used to generate predictable out-of-frame deletions and to incorporate donor DNA sequences in a highly efficient manner (25).
We previously presented the web-based Mojo Hand designer tool (26). In the latest version 2.0, algorithm adheres to the same general steps that the original algorithm follows with the integration of new features including .bed file creation, microhomology and out-of-frame scoring. Another major consideration was the incorporation of user generated next generation sequencing data in reagent design to deal with the tremendous inter- and intrastrain genetic variation during zebrafish genome targeting. In the current version, high-depth RNAseq datasets were integrated to simplify design and reduce time and cost through the avoidance of regions rich in single nucleotide polymorphisms (SNPs). Here, we describe a detail protocol of targeted zebrafish genome editing through NHEJ and HDR, respectively, using TALENs or CRISPR/Cas9 using the open access Mojo Hand 2.0 software.
2. Materials
2.1 Zebrafish embryo genotyping and RFLP assay
Genomic DNA extraction buffer: 10 mM Tris-HCl, pH 8.3, 50 mM KCl
10% Tween-20
10% NP-40
Proteinase K solution (Recombinant, PCR Grade, 14–22 mg/mL in 10 mM Tris-HCl, pH 7.5, Roche Life Science)
PCR reaction mix (see Note 1)
Restriction enzyme
Agarose
TAE Buffer (1×): 40 mM Tris-HCl, 20 mM acetic acid, 1 mM EDTA, pH 8.4
Standard gel Electrophoresis system
2.2 TALEN Assembling
-
1
FusX collection (pFusX1 – 4 and pFusX_B2) (Addgene in progress)
-
2
Last half-repeat components pLR-NI, -HD, -NN, and -NG (Addgene #31006, #30984, #31017, #30995)
-
3
RCIscript-GoldyTALEN backbone (Addgene, cat# 38142)
-
4
T4 DNA Ligase (2,000,000 units/mL, New England Biolabs)
-
5
BsmBI (New England Biolabs) (optional, see Note 2)
-
6
Esp3I (Thermo Scientific)
-
7
Standard thermocycler
-
7
Competent E. coli cell
-
8
LB agar plate with ampicillin (100 μg/mL)
-
9
LB medium with ampicillin (100 μg/mL)
-
10
20 mg/mL X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactoside)
-
11
0.1M IPTG (isopropylthio-β-galactoside)
-
12
Colony PCR screening primers: TAL_F1 (ttggcgtcggcaaacagtgg) and TAL_R2 (ggcgacgaggtggtcgttgg) (23)
-
13
Sequencing primers: TAL_F1, TAL_R2, RVD-MM-F (ctcacacccgatcaggtc) and RVD-MM-R (gacctgatcgggtgtgag) (see Note 3) (24)
2.3 In vitro Transcription
SacI (New England Biolabs)
3 M sodium acetate, pH 5.0
70% ethanol
Ambion mMESSAGE mMACHINE® T3 Transcription Kit (Life Technologies)
Deionized water
Lithium Chloride precipitate solution: 7.5 M LiCl, 50 mM EDTA, pH 8.0
3. Methods
3.1 Designing TALEN with Mojo Hand 2.0 (Fig. 1, see Note 4)
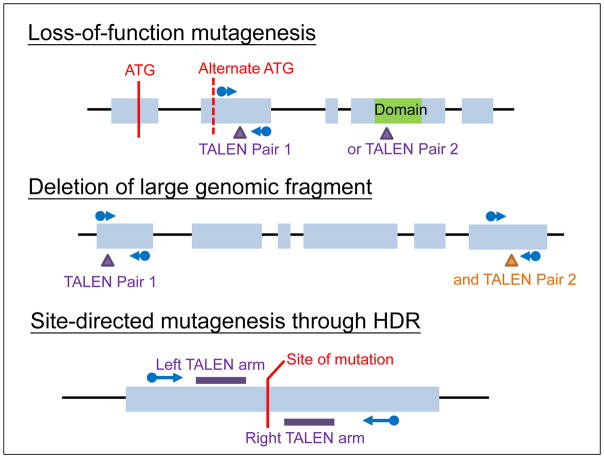
Figure 1.
Typical genomic region for TALEN targeting in different types of mutagenesis. Either TALEN Pair 1 or 2 can be used in case of loss-of-function mutagenesis and TALEN pair 1 and 2 are used together for deletion of large genomic fragment. Blue arrow indicated primer pairs for RFLP or PCR screening of mutagenesis.
Select genomic region for TALEN targeting (Fig. 2, see Note 5)
Sequence Input into Mojo Hand 2.0 (http://talendesign.org/)
-
Identification of Binding Sites with the following parameters (Note 6):
Length of TAL binding domain 15RVDs
Spacer length between 14–18 bp
Unique restriction site within the spacer for RFLP assay of NHEJ-mediated mutagenesis (optional for large genomic fragment deletion using two pairs of TAELN, see Note 7)
T nucleotide upstream of both TAL binding domains
Restriction enzyme analysis
Mojo Hand Output
Select TALEN design with desired Microhomology Score above or Out-of-frame Score if predictable deletion through MMEJ is desirable (Note 8)
Generate BED File to be used in conjunction with Integrated Genomics Viewer (IGV) (Note 9)
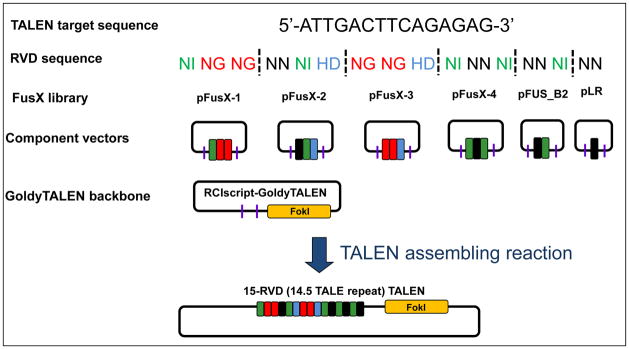
Figure 2.
Picking corresponding component vectors from FusX libraries to assembly 15-RVD GoldyTALEN
3.2 Genotyping targeted genomic locus
Design primers to amplify the targeted locus (see Note 10).
-
Extract genomic DNA from zebrafish embryos of the targeted fish line (see Note 11)
To prepare 1 mL working extraction buffer, freshly add 30 μL 10% Tween-20, 30 μL 10% NP-40 and 10 μL Proteinase K to 950 μL genomic DNA extraction buffer
Transfer embryos to centrifuge tube and remove excess embryo water
Add working extraction buffer (50 μL per embryo)
Incubate at 55 °C with shaking ≥ 4 hours
Incubate at 98 °C for 10 min to inactivate Proteinase K
Store genomic DNA at −20 °C until PCR
Typically, 5 μL of genomic DNA solution are used in 25 μL PCR reaction
PCR amplify the target locus
-
Test RFLP assay
Confirm sequence of the targeted locus by Sanger Sequencing and identify any polymorphic region affecting TALEN binding sites, re-design TALEN if necessary.
3.3 Design short single-stranded donor oligo
-
Design donor oligo with the following parameters:
Around 50 base pairs in length
Mutated nucleotide(s) in the middle part of the oligo
Unique restriction site added in the middle of the oligo by introducing silent mutations to allow easy screening of donor incorporation with RFLP assay
3.4 TALEN Assembling with FusX System (3-days)
3.4.1 Day 1
-
1
Breakdown the 15-RVD TALE domain from 5′ to 3′ into 6 building blocks from different libraries of the FusX kit according to the formula 3 (pFusX-1) + 3 (pFusX-2) + 3(pFusX-3) + 3 (pFusX-4) + 2 (pFus_B2) + 1 (pLR) (Fig. 2)
For example:
A TALEN arm with the following targeting sequence: 5′-ATTGACTTCAGAGAG-3′
Corresponding RVD sequences: NI NG NG NN NI HD NG NG HD NI NN NI NN NI NN
List of building blocks required for each TAL:
| Library | RVD sequence |
|---|---|
| pFusX-1 | NI NG NG |
| pFusX-2 | NN NI HD |
| pFusX-3 | NG NG HD |
| pFusX-4 | NI NN NI |
| pFus_B2 | NN NI |
| pLR | NN |
-
5
Mix 25–50 ng of each vector in a PCR tube with 50 ng RCIscript-GoldyTALEN backbone (see Note 12)
-
6
(Optional) Add to each reaction, 1 μL 10X NEBuffer 3.1, 0.5 uL BsmBI and make up to 10 μL with deionized water (see Note 2)
-
7
(Optional) Incubate at 55ºC for 30 min (see Note 2)
-
8
Add to each reaction 1.5 μL 10X T4 DNA Ligase Reaction Buffer, 0.5 μL T4 DNA Ligase, 0.5 μL Esp3I and make up to 15 μL with deionized water
-
9
Run the follow program in thermocycler:
37 °C, 5 min and 16 °C, 10 min → 10 cycles,
37°C, 15 min,
80°C, 5 min,
4°C forever
-
10
Transform 3–5 μl of the reaction product and plate ~1/5 of the recovered transformants on LB agar plate with ampicillin, 40 μL X-Gal (20 mg/mL) and 40 μL 0.1M IPTG
-
11
Incubate LB agar plate at 37°C overnight
3.4.2 Day 2
Pick 2–4 white colonies for colony PCR with primers TAL_F1 and TAL_R2
-
PCR with the follow program (see Note 13)
95 °C, 10 min
95 °C, 30 s; 55 °C, 30 s and 72°C, 3 min → 30 cycles
72°C, 5 min
4°C forever
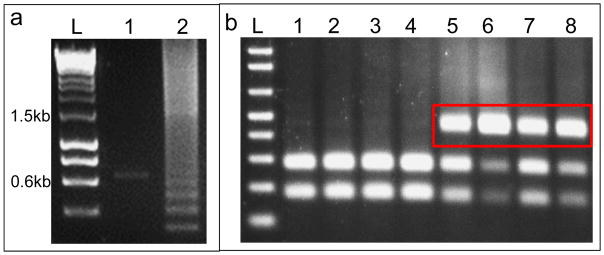
Resolve PCR product in 1% agarose gel and identify positive clones (Fig. 34a)
Culture positive colonies overnight 37°C in LB with ampicillin
3.4.3 Day 3
Mini-Prep overnight cultures of selected positive clones
Verify assembled TALEN by Sanger sequencing with TAL_F1 and TAL_R2 (see Note 3)
3.5 Synthesizing TALEN encoding mRNA and microinjection into 1-cell zebrafish embryos
Linearize TALEN encoding plasmid with SacI
Purify linearized plasmid by ethanol precipitation and quantify purified plasmids
Set up in vitro transcription reaction with Ambion mMESSAGE mMACHINE® T3 Transcription Kit according to manufacturer’s instruction (see Note 14)
-
Purify and quantify transcribed mRNA
Add 50 μL LiCl Precipitation Solution to each transcription reaction
Precipitate at −20 °C ≥ 1 hour
Centrifuge at 4 °C, 12,000g for 15 min
Remove supernatant and wash with 70% ethanol
Centrifuge at 4 °C, 12,000g for 5 min
Remove supernatant and air dry pellet
Resuspend pellet in 50 μL deionized water and quantify mRNA
Make working stock for microinjection by mixing and diluting both mRNA encoding the TALEN pair (final concentration ~20 ng/μl of each TALEN mRNA, 20pg × 2, see Note 15)
Microinject (20 pg to 100 pg each TAELN arm, see Note 16) into the yolk of 1-cell embryos
3.6 Examine somatic TALEN activity by RFLP assay or PCR to detect a large deletion
Extract genomic DNA from control (uninjected) and TALEN injected embryos (see Note 17) as described in section 3.2
PCR amplify the target locus
Digest 10 μL PCR product with appropriate restriction enzyme and resolve digested product on 1.5% agarose gel (Fig. 34b)
To detect a large deletion generated by two TALEN pairs, extract genomic DNA from control (uninjected) and TALEN injected embryos (see Note 18) as described in section 3.2
PCR amplifies the target locus with appropriate primers (see Note 18, Fig. 2) and resolve PCR product on agarose gel
3.7 Screening of germline transmission for stable mutants
For loss-of-function mutagenesis using a single TALEN pair, germline transmission efficiency correlated with TALEN mutagenic activity. Usually founder fish will be identified within screening of 10 injected fishes when working with a moderately active TALEN (~60% mutagenic activity in RFLP assay). In large deletion with 2 TALEN pairs, efficiency is typically 2–5 fold lower, also depending on activity of TALEN pairs. In case of site-directed mutagenesis through HDR, efficiency will be ~100 fold lower and a much larger number of injected fishes will have to be screened for founder.
Raise potential batches of injected embryos (siblings showing expected somatic mutation)
Genotype juvenile fishes (around 4–6 weeks old) by tail fin biopsy (see Note 19)
Extract genomic DNA from fin tissue following section 3.2 and screen with RFLP or PCR assay for maintenance of induced as described in section 3.6
Raise juveniles with stable somatic mutations to sexually mature and out-cross with Wild-type to obtain F1 embryos
Extract genomic DNA from individual F1 embryos following section 3.2 and genotype with RFLP or PCR assay
Raise potential batches of F1 embryos (siblings showing heterozygous mutation)
Genotype juvenile F1 as described in step 2–3
Confirm mutation carried in F1 by Sanger sequencing (see Note 20)
Figure 3.
(a) Typical colony PCR result after TALEN assembling. Lane 1 is a negative clone with empty GoldyTALEN backbone showing a ~0.65kb band and Lane 2 is a positive TALEN clone showing the laddering effect with a band at ~1.5kb. (b) Typical RFLP assay result of single embryos. Lane1–4 are uninjected control with completely digested PCR product and Lane 5–8 are embryos injected with TALEN showing undigested products (red box). L: Ladder.
Acknowledgments
We thank all of the great user comments for refining this genome engineering design system. This project is supported by the State of Minnesota (University of Minnesota/Mayo Clinic Gene Targeting Partnership grant H001274506-3 to SCE), National Institutes of Health grants GM63904 and P30DK084567 to SCE, the Mayo Foundation, the Health and Medical Research Fund (HMRF02132326) to ACM and HKU Seed Funding for Basic Research (201401159004, 201411159098) to ACM.
Footnotes
Any PCR reagents could be used and ready-to-use PCR master mix will be efficient in high throughput screening.
BsmBI and Esp3I are isoschizomers which have different optimum reaction temperature (55 °C and 37 °C, respectively). While it is not recommended to used in cycling reaction with T4 DNA Ligase, optional pre-digestion with BsmBI at 55 °C will significantly enhance the efficient of TALEN assembly, reducing the number of blue colonies.
For TALEN with 15-RVDs, Sanger sequencing with TAL_F1 and TAL_R2 will typically cover all 14.5 repeat units. In case unit(s) are missed in sequencing, RVD-MM-F and RVD-MM-R, with sequence specific to RVD-8 can be used.
Mojo Hand is available as a web service at www.talendesign.org. The site allows access to the program without the trouble of installation and with the ease of a familiar interface. Point-of-use help is available for each field. The source code and spreadsheet are also available for non-commercial use with applicable license.
For loss-of-function mutation, TALEN should be designed against early conserved exons after the start codon (and alternate start codon) or important functional domain such that small indels will be introduced through NHEJ and resulted in frame-shifting / pre-mature termination. For deletion of a large genomic fragment with two pairs of TALEN, simply design two pairs of TALEN flanking the genomic fragment to be deleted. For site-directed mutagenesis through HDR, TALEN should target the site to be mutated.
Templates for each system can be changed to user specifications. Notation for templates has been slightly changed from “.” representing a non-preferential base to “N” representing any base. The default template for TALENs remains TsN*e, which constrains TAL binding sites to an initial 5′ T bp.
For deletion of a large genomic fragment with two pairs of TALEN, unique restriction site in spacer for RFLP assay is not necessary since deletion can be simply detected by PCR (see Note 5). However, inclusion of restriction site in the design of both TALEN pairs is recommended such that activity of each TALEN pair can be confirmed with RFLP assay before co-injection.
Microhomology-mediated end joining (MMEJ) is a Ku- and ligase IV independent DNA repair mechanism that utilizes regions of microhomology adjacent to the site of DSB. Because in-frame deletions can sometimes lower the efficiency of loss-of-function mutagenesis, we integrated an algorithm developed by Bae et al (25) into Mojo Hand that calculates a Microhomology Score and an Out-of-frame Score for each binding site. The Microhomology Score is an aggregate of each pattern score associated with each microhomology between two to eight bases long, and the pattern score is calculated based on the length of the microhomology and deletion. Higher Microhomology Scores correspond with binding sites with stronger microhomologies. Out-of-frame Score is the percentage of Microhomology Score from frame-shifting microhologies for each binding site. Predicted deletions gives a list of all homologies within a binding site, with their sequences, deletion lengths, pattern scores, and whether or not they cause frameshifts. Higher pattern scores correlate with a higher chance of any particular deletion occurring due to microhomology-mediated end joining. This prediction does not take into account deletions that occur due to NHEJ.
Integrated Genomics Viewer (IGV) is a tool that allows users to visualize their own genomic data sets and load tracks and other features in a variety of formats. We utilized the BED file format to store user designs for site-specific nucleases, which can then be loaded as a searchable feature within the track line of IGV. This allows users to visualize potential TALEN candidates in tandem with their own in-house next generation sequencing data sets in an efficient and intuitive manner. BLAT search maps each potential binding site across the genome, which allows users to visualize and avoid designs that are not unique. In addition this function can be used to avoid designs that bind within polymorphic stretches of the genome that may negatively impact cutting efficiency. BED files are created by using the BLAT tool (27) to map binding sites and restriction enzymes to a genome specified by the user. Current genomes supported by Mojo Hand include D. rerio and C. elegans due to current hosting limitations. A detailed specification of BED file format is available at http://genome.ucsc.edu/FAQ/FAQformat.html#format1.
Although there is no restriction on primer design for initial genotyping purposes, primer pair can be designed such that they could also be used for RFLP assay. Typically, primers with amplicon sized around 300 to 500 base pairs work well for RFLP assay. Avoid having the unique restriction site for RFLP assay in the middle of the amplicon, which otherwise, would give two similar sized digestion products difficult to be resolved in electrophoresis.
To identify potential polymorphic region, genomic DNA can be extracted different batches of non-sibling embryos.
Assembling reaction works well even if component vectors varied in amounts within range. Equal volume of each vector could be mixed to simplify reaction setup even if their concentrations are different.
PCR cycle can be further optimized based on the PCR reagent used.
An initial 10 μL half in vitro transcription reaction resuspended in 25 μL final volume will typically yield mRNA at concentration around 500–1000 ng/μL, which is more than enough in most applications.
Working mRNA solution should be stored in small aliquots and avoid repeated freeze-thaw.
It is recommended to conduct dose-response trials within the range from 20 to 100 pg per TALEN arm such that the optimum dose can be chosen which resulted in survival of around 50% of normally developed embryos.
Genomic DNA could be extract from single embryo to examine mutagenic activity in individual embryo or from a group of 5 or 10 embryos to assay the average mutagenic activity of the TALEN.
For screening large genomic deletion, forward primer used to genotype TALEN pair 1 and reverse primer used TALEN Pair 2 can be used together to screen for large deletion with a smaller sized PCR product compare with the larger or absent PCR product in control. Reverse primer from Pair 1 and forward primer from Pair 2 can also be used together to screen for very rare “flipping” event where the targeted genomic fragment was excised but inversely inserted back into the genomic lesion. Since the PCR screening is only qualitative and does not reflect mutagenic activity, genomic DNA can be extracted from single embryo instead of a group of embryos.
This round of fin biopsy is optional. However, pre-screening for stable somatic mutation will significantly increase the percentage of founder in the pool. Therefore, it is recommended in case of large fragment deletion and site-directed mutagenesis, where germline transmission efficiency is considerably lower.
F1 carrying desirable mutation will be selected. For example, small indels resulted in frame-shifting or pre-mature stop in case of loss-of-function mutagenesis and precisely incorporated donor sequence in site-directed mutagenesis.
References
- 1.Bai J, Choi SH, Ponciano G, et al. Xanthomonas oryzae pv. oryzae avirulence genes contribute differently and specifically to pathogen aggressiveness. Mol Plant Microbe Interact. 2000;13:1322–1329. doi: 10.1094/MPMI.2000.13.12.1322. [DOI] [PubMed] [Google Scholar]
- 2.Yang B, White FF. Diverse members of the AvrBs3/PthA family of type III effectors are major virulence determinants in bacterial blight disease of rice. Mol Plant Microbe Interact. 2004;17:1192–1200. doi: 10.1094/MPMI.2004.17.11.1192. [DOI] [PubMed] [Google Scholar]
- 3.Boch J, Scholze H, Schornack S, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 4.Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 5.Christian ML, Demorest ZL, Starker CG, et al. Targeting G with TAL Effectors: A Comparison of Activities of TALENs constructed with NN and NK Repeat Variable Di-Residues. PLoS One. 2012;7:e45383. doi: 10.1371/journal.pone.0045383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Streubel J, Blücher C, Landgraf A, et al. TAL effector RVD specificities and efficiencies. Nat Biotechnol. 2012;30:593–595. doi: 10.1038/nbt.2304. [DOI] [PubMed] [Google Scholar]
- 7.Miller JC, Tan S, Qiao G, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 8.Bedell VM, Wang Y, Campbell JM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F, Cong L, Lodato S, et al. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol. 2011;29:149–153. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cong L, Zhou R, Kuo YC, et al. Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains. Nat Commun. 2012;3:968. doi: 10.1038/ncomms1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crocker J, Stern DL. TALE-mediated modulation of transcriptional enhancers in vivo. Nat Methods. 2013;10:762–767. doi: 10.1038/nmeth.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeder ML, Angstman JF, Richardson ME, et al. Targeted DNA demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins. Nat Biotechnol. 2013;31:1137–1142. doi: 10.1038/nbt.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendenhall EM, Williamson KE, Reyon D, et al. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013;31:1133–1136. doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thanisch K, Schneider K, Morbitzer R, et al. Targeting and tracing of specific DNA sequences with dTALEs in living cells. Nucleic Acids Res. 2014;42:e38. doi: 10.1093/nar/gkt1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hockemeyer D, Wang H, Kiani S, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731–733. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesson L, Usal C, Ménoret S, et al. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011;29:695–696. doi: 10.1038/nbt.1940. [DOI] [PubMed] [Google Scholar]
- 17.Carlson DF, Tan W, Lillico SG, et al. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci USA. 2012;109:17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Li C, Yu Z, et al. Efficient and specific modifications of the Drosophila genome by means of an easy TALEN strategy. J Genet Genomics. 2012;39:209–15. doi: 10.1016/j.jgg.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang F, Li X, et al. Transcription activator-like effector nucleasese enable efficient plant genome engineering. Plant Physiol. 2013;161:20–27. doi: 10.1104/pp.112.205179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma S, Zhang S, Wang F, et al. Highly Efficient and Specific Genome Editing in Silkworm Using Custom TALENs. PLoS One. 2012;7:e45035. doi: 10.1371/journal.pone.0045035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung YH, Baek IJ, Kim DH, et al. Knockout mice created by TALEN-mediated gene targeting. Nat Biotechnol. 2013;31:23–24. doi: 10.1038/nbt.2477. [DOI] [PubMed] [Google Scholar]
- 22.Ma AC, Lee HB, Clark KJ, et al. High efficiency in vivo genome engineering with a simplified 15-RVD GoldyTALEN design. PLoS One. 2013;8:e65259. doi: 10.1371/journal.pone.0065259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cermak T, Doyle EL, Christian M, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng Y, Clark KJ, Campbell JM, Panetta MR, Guo Y, Ekker SC. Making designer mutants in model organisms. Development. 2014;141:4042–4054. doi: 10.1242/dev.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae S, Kweon J, Kim SK, et al. Microhomology-based choice of Cas9 nuclease target sites. Nature Methods. 2014;11:705–706. doi: 10.1038/nmeth.3015. [DOI] [PubMed] [Google Scholar]
- 26.Neff KL, Argue DP, Ma AC, et al. Mojo hand, a TALEN design tool for genome editing applications. BMC Bioinformatics. 2013;14:1. doi: 10.1186/1471-2105-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kent WJ. BLAT - the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]