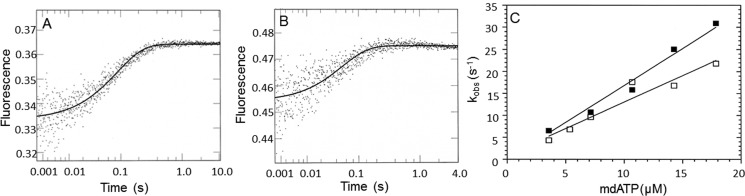
Figure 4.
dmantATP binding to myoVIIa-sh1 and actomyoVIIa-sh1. 1.1 μm myoVIIa-sh1 (A) and 0.7 μm actomyoVIIa-sh1 (0.7 μm myosin and 1.4 μm actin) (B) were mixed with 7.2 and 10.7 μm dmantATP, respectively. The increase in fluorescence was recorded, and three traces were averaged and fit to a single exponential equation, resulting in a kobs of 10.7 s−1 (A) and 17.7 s−1 (B). Experimental conditions in the cell were as follows: 1.1 μm myoVIIa-sh1 (A), 0.7 μm myosin and 1.4 μm actin (B), 5.5 μm CaM (A) and 3.5 μm CaM (B), 10 mm MOPS, 3 mm MgCl2, 1 mm EGTA, 25 mm KCl, 1 mm DTT, pH 7.5, 20 °C. C, experimental conditions were the same as in A and B except that 1.8–18 μm dmantATP was used. The data were fit to a linear equation and resulted in apparent second order association rate constants of kT = 1.7 μm−1 s−1 ± 0.07 and kAT = 1.2 μm−1 s−1 ± 0.14 for dmantATP binding to myoVIIa-sh1 (■) and actomyoVIIa-sh1 (□), respectively.