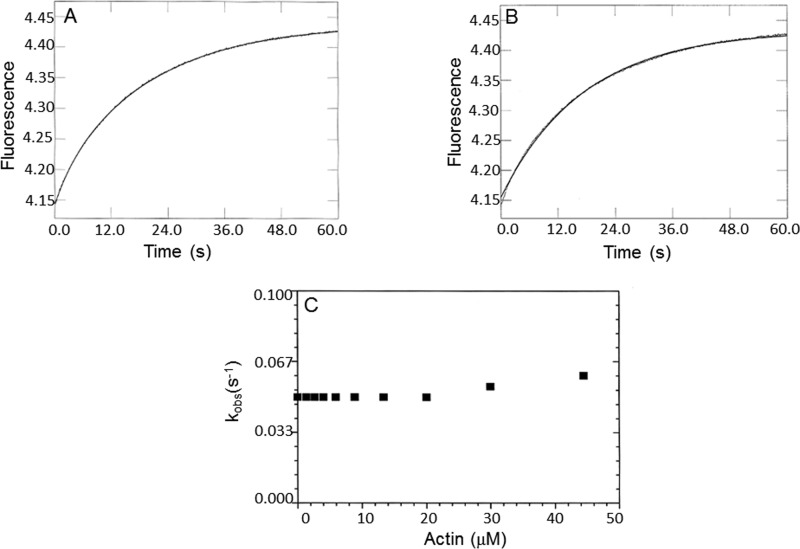
Figure 9.
Phosphate dissociation from the actomyoVIIa-sh1-ADP-Pi complex. Double mixing stopped-flow experiments were performed using MDCC-PBP. MyoVIIa-sh1 was first mixed with ATP, held in a delay line for 10 s, and then mixed with actin to accelerate Pi release. Final concentrations in the flow cell were 0.8 μm myoVIIa-sh1, 4 μm CaM, 0.4 μm ATP, 0–44 μm actin, 1 mm EGTA, 3 mm MgCl2, 10 mm MOPS, 25 mm KCl, 10 μm MDCC-PBP, 0.1 mm 7-methylguanosine, and 0.01 unit/ml purine nucleoside phosphorylase, pH 7.5, 20 °C. A, phosphate dissociation from the 0.8 μm shaker-1-ADP-Pi complex in the presence of 20 μm actin. A single exponential equation fit through the data resulted in I(t) = 0.28e−0.06 + C. B, a biexponential fit to the data resulted in I(t) = 0.03e−0.29 + 0.26e−0.05. C, dependence of the kobs of the slow phase on actin concentrations resulted in a k−DAP ≥ 0.05 s−1.