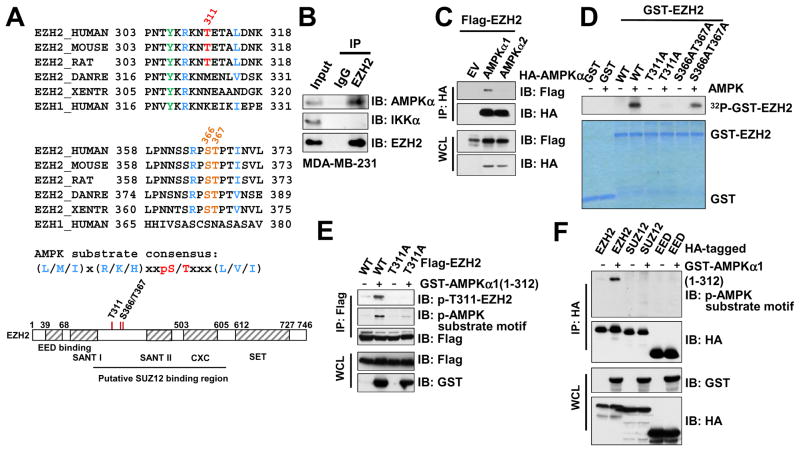
Figure 3. Identification of EZH2 as a Downstream Phosphorylation Substrate of AMPK.
(A) Sequence alignments of the T311- and the S366/T367-containing regions between EZH2 among various species as well as human EZH1. A schematic representation of EZH2 protein domain structure was shown beneath to illustrate the position of these candidate sites.
(B) Immunoblot (IB) analysis of whole cell lysates (WCL) and anti-EZH2 immunoprecipitates (IP) derived from MDA-MB-231 cells.
(C) IB analysis of WCL and anti-HA IP derived from 293T cells transfected with Flag-EZH2 and the indicated HA-AMPKα constructs.
(D) In vitro kinase assays showing that bacterially purified recombinant WT-EZH2, and to a much lesser extent, S366A/T367A-EZH2, but not the T311A-EZH2 mutant, could be phosphorylated by the AMPK kinase in vitro.
(E) AMPK primarily phosphorylated EZH2 at T311. IB analysis of WCL and anti-Flag IP derived from 293T cells transfected with the indicated constructs.
(F) IB analysis of WCL and anti-HA IP derived from 293T cells transfected with the indicated HA-EZH2, HA-SUZ12 and HA-EED constructs.
See also Figure S3.