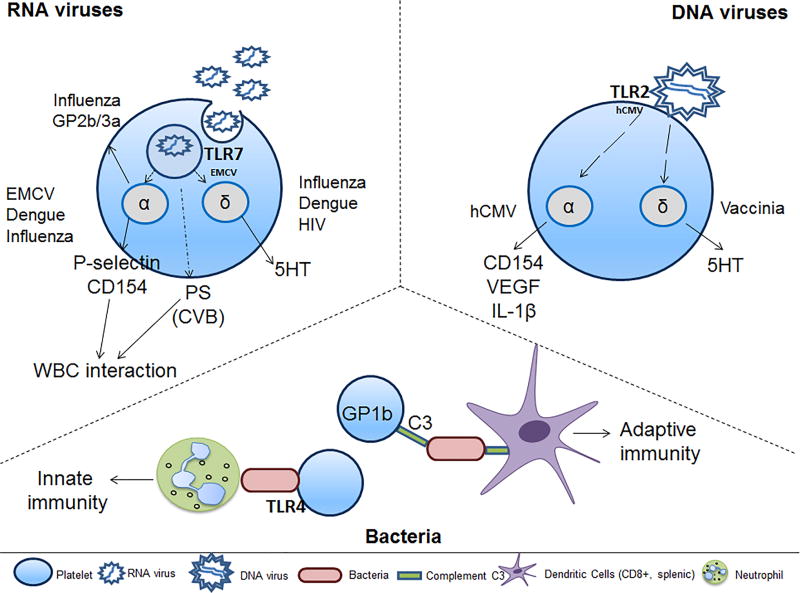
Figure 3. Platelet and circulating cell interactions during infection initiate the innate or adaptive immune response.
Platelets achieve cell-to-cell communication during bacterial or viral infection either by direct interaction with WBCs through surface expression of platelet proteins or through indirect protein release from their α- or δ-granules. EMCV-activated platelets interact with neutrophils in a TLR7-dependent manner. CVB-activated platelets bind to neutrophils in a PS-dependent manner. Dengue and influenza increase microparticle release; dengue-mediated microparticles contain IL-1β. HCMV-activated platelets interact with neutrophils, monocytes, B-cells, T-cells and DCs, suggesting activation of innate and adaptive immune responses. Vaccinia-bound platelets have reduced aggregation potential in the presence of ADP, collagen, or thrombin. The specific pathways by which platelets respond to Herpes simplex virus (HSV)1 or HSV2 are currently unknown. During bacterial infection, platelet interactions with complement C3 opsonized bacteria through GP1b (CD42) lead to slowing of bacterial clearance. DCs recognize the platelet-bacterial complexes, thereby, inducing adaptive immunity. These platelet-bacterial interactions are true for Gram-positive or Gram-negative bacteria. Abbreviations: WBC-white blood cells; 5HT-serotononin; VEGF-vascular endothelial growth factor; PS-phosphatidylserine; C3-complement C3; EMCV-encephalomyocarditis virus; hCMV-human cytomegalovirus; CVB-coxsackievirus B; HIV-human immunodeficiency virus;