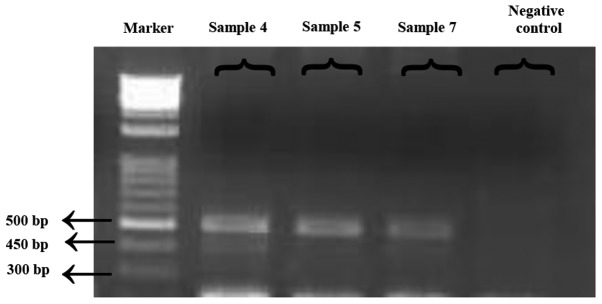
Figure 2.
Data on the detected E255K mutations in four patients. For the detected mutations, ASO-PCR products resolved via agarose gel electrophoresis are presented. Marker, DNA ladder 100 bp. Sample 4: PCR-amplified product of the E255K mutation using gDNA from a patient with CML receiving glivec treatment. Sample 5: PCR-amplified product of the E255K mutation using gDNA from a patient with CML receiving nilotinib treatment. Sample 7: PCR-amplified product of the E255K mutation using gDNA from a patient with CML receiving imatinib treatment. Negative control: One peripheral blood sample was obtained from a patient with CML exihibiting wild type for ABL mutations and used as a negative control. ASO-PCR, allele-specific oligonucleotide polymerase chain reaction; CML, chronic myeloid leukemia; gDNA, genomic DNA.