Abstract
Objectives
This study addresses methodological and theoretical questions about the association between affect and physical health. Specifically, we examine the role of affect variability and its interaction with mean levels of affect to predict antibody (Ab) levels in response to an influenza vaccination.
Methods
Participants (N = 83) received the vaccination and completed daily diary measures of affect four times a day for 13 days. At one and four months post-vaccination, blood was collected from the participants to assess Ab levels.
Results
Findings indicate that affect variability and its interaction with mean levels of affect predict an individual’s immune response. Those high in mean positive affect (PA) who had more PA variability were more likely to have a lower Ab response in comparison to those who had high mean PA and less PA variability. Although it did not interact with mean negative affect (NA), NA variability on its own was associated with Ab response, whereby those with less NA variability mounted a more robust immune response.
Conclusion
Affect variability is related to immune response to an influenza vaccination and, in some cases, interacts with mean levels of affect. These oscillations in affective experiences are critical to consider in order to unpack the intricacies of how affect influences health. These findings suggest that future researchers should consider the important role of affect variability on physical health-relevant outcomes as well as examine the moderating effect of mean affect levels.
Keywords: affect variability, antibody response, immune function, negative affect, positive affect
Introduction
Positive affect (PA), such as feelings of joy or happiness, has been repeatedly tied to better health and physiological function [1–3], while the converse is true of negative affect (NA; e.g., feelings of sadness or anger; [4]). The majority of this research has evaluated affect in a singular fashion: by assessing mean or average levels of affect. This ignores the interesting possibility that naturally occurring changes in affect over time, uncaptured by averages, might also have biological relevance [5].
Fluctuations in the experience of affect over time are referred to as affect variability. This construct captures the idea that an individual who varies between extreme highs and lows on NA, for example, is starkly different from an individual with consistently moderate levels of NA. These two individuals could have the same mean level of NA, however, and would therefore be considered equal in many past studies about state affect and physical health (see Figure 1). Without consideration of variability, invaluable information about nuances in affect is lost. Critically, with knowledge of the interplay between transient affect and alterations in physiology (e.g., heart rate, blood pressure, immune function; [6, 7]), it seems plausible that these variability differences may have physical health-relevant consequences.
Figure 1.
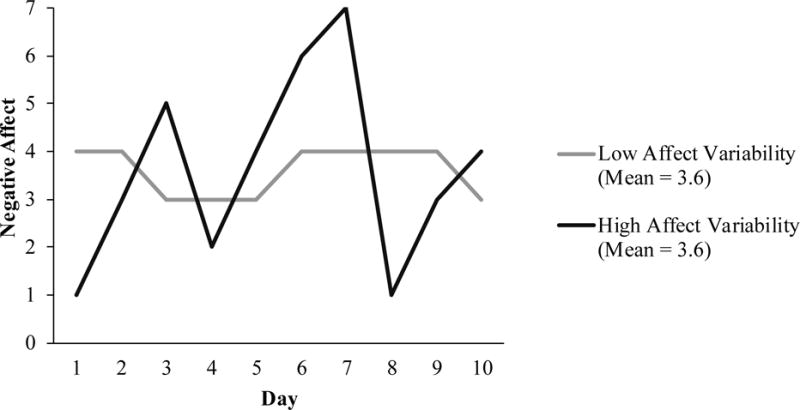
Two individuals with the same mean level of negative affect but different negative affect variability.
A substantial body of evidence suggests that affect variability may be associated with worse mental health (see meta analytic review [8]). For example, Gruber and colleagues [9] found that greater PA variability was associated with lower life satisfaction, worse psychosocial functioning, and greater depression and anxiety. These findings held even when controlling for mean affect, indicating that variability may predict mental health over and above mean levels of affect. In the same paper, retrospectively captured affect variability in a separate large sample showed that greater PA variability was associated with lower life satisfaction and subjective happiness. Similar to these findings, Hardy and Segerstrom [10] found that middle-aged participants with greater variability in both PA and NA experienced greater psychological distress even when controlling for each respective mean level of affect. These findings indicate that greater affect variability is detrimental to mental health.
While this evidence provides convincing support that affect variability has implications for psychological health, there is a near absence of work examining how affect variability may impact physical health-relevant outcomes. Indeed, variability in other psychological characteristics (e.g., life satisfaction, perceived control) is associated with a variety of physical health outcomes such as higher mortality risk and worse physical health, and physical healthrelevant factors such as lower social support [11–13]. There is also the possibility that variability in affect has a physiologically taxing effect on the body. For example, given the known cardiovascular, immune, and hormonal alterations with even subtle affect change (e.g., [14–17]), variability may take additional energy due to repeated physical adjustments. Alternatively, one could argue that variability is healthful given that it gives activated physiological systems a break, which prevents biological exhaustion and wear and tear on these systems (e.g., [18, 19]). Although these predictions suggest affect variability may be tied to physical health, a surprising aspect of the current variability literature is the lack of inclusion of objective health-relevant biomarkers. To our knowledge, only one study has examined the association between affect variability and a health-relevant biomarker, finding that moderate levels of PA variability were related to daily cortisol profiles that are reflective of better physiological functioning [20]. If we are to better understand the toll affect variability takes on physical health, we must continue to study objective markers of health.
One health-relevant biomarker that may be important in regard to affect variability is antibody (Ab) response to a vaccination, such as the influenza vaccine. Ab response, typically assessed via blood samples, is often used to study how psychosocial factors impact in vivo immune function [21–23]. Given the importance of a quick and large rise in Ab to ensure protection against virus exposure [22], vaccination response provides us with a health-relevant indicator of immune functioning. For the influenza vaccine, Ab increases one month post-vaccination represent the maximum response, while Ab levels after that time represent the extent to which the Ab increase is sustained versus declined (e.g., [24]). Critically, affect variability experienced immediately following vaccination might have physiological implications that may be associated with these Ab levels.
In addition to the limitation of the lack of health-relevant biomarkers, previous affect variability and health research has also not included interaction terms between affect variability and mean levels of affect. This may be important because variability may have different implications based on mean levels [25]. For example, an individual with high mean PA may benefit from low variability because he or she would experience consistently high levels of PA. On the other hand, an individual low on mean PA may benefit from high variability because he or she could at least experience some instances of high PA, which could provide temporary benefits. However, this also means that he or she will be experiencing instances of extremely low PA when he or she drops far below his or her already low PA level. For NA, similar instances could occur. Individuals with high mean NA may benefit from high variability because this provides “breaks” in NA (when they drop below their usually high NA levels), while those low in mean NA may benefit from low variability so that they stay consistently low on NA. As noted by these examples, the combination of these potential interaction effects may have profound effects on how affect influences health. Although affect papers have not tested this interaction, one study investigated the interaction between variability and mean levels of life satisfaction (which is only moderately correlated with affect [26]) and showed that greater variability was associated with an increase in mortality risk, especially for those with low mean life satisfaction [11].
The goal of the present study is to examine how affect variability is associated with Ab response to an influenza vaccination. This study fills important gaps in the literature by employing a fine-grained methodology to assess affective experiences, measuring a novel healthrelevant biomarker that provides rich information about immunocompetence, and examining previously unexplored interaction effects. Affect variability was measured using the common standard deviation approach (similar to the methods used by the papers reviewed above). This method is advantageous in that it represents affect variability with a single value that is widely used and understood [27–29]. We interacted mean affect with affect variability to uncover whether affect variability has different implications for physical health at different levels of mean affect.
Method
Participants
Participants included 83 undergraduate students (Mage = 18.29; SDage = 0.90; 44% male). Sixty-six percent were Caucasian, 24% were Asian, and 10% were other or mixed ethnicity. Participants were eligible for participation if they were healthy (i.e., no chronic or acute illnesses), were not on a regular medication regimen (with the exception of birth control), had never been vaccinated for influenza, and were not pregnant or breastfeeding. Participants were compensated $120. All study procedures were approved by the university Institutional Review Board.
Procedures
Participants were run in two cohorts across the fall in consecutive years. Participation in the study lasted for four months. Participants first completed baseline measures and then completed daily diaries four times a day for 13 consecutive days. Data were collected on a handheld computer which alerted participants to complete questionnaires one hour after their wake time and then three, eight, and 10 hours later. On day three, participants received the flu vaccination at a university flu clinic. Before receiving the vaccination, blood was collected to measure Ab levels. At one and four months post-vaccination, blood was again collected from the participants to assess Ab levels.
Measures
Daily affect
Affect was assessed with a checklist of 12 adjectives adapted from the State Adjective Questionnaire [30, 31]. Participants reported how much each adjective represented their current affect at each of the diary entries. NA was assessed with the items jittery, nervous, unhappy, and sad. PA was assessed with the items active, intense, enthusiastic, lively, happy, cheerful, relaxed, and calm. NA and PA items were rated on a scale from 0 (Not at all) to 4 (Extremely). Cronbach’s alphas for NA ranged from .56 to .84 and Cronbach’s alphas for PA ranged from .68 to .85 across the four time points over 13 days.
Affect variability and mean affect
For the purposes of examining how affect variability after the vaccination influenced Ab response, only those days on or after the flu vaccine (i.e., days 3 through 13) were used to create the affect variability values1. Therefore, adjective items were averaged over each of the 44 time points (11 days* 4 assessments) to create a NA and PA mean value (split-half reliabilities were 0.91 and 0.90, respectively). Then, standard deviations over the 44 time points were calculated for NA and PA (split-half reliabilities were 0.67 and 0.68, respectively; reliability estimates reflect similar ones reported in previous affect variability literature [32, 33]). These calculations resulted in the following variables used for analyses: NA mean (NAMEAN), PA mean (PAMEAN), NA standard deviation (NASD), and PA standard deviation (PASD).
Antibody response to vaccination
Ab levels were assessed using venipuncture blood sampling. A 20-mL sample of blood was collected immediately before the immunization occurred (i.e., baseline) and at one and four month follow-up appointments. The Fluzone vaccine consisted of three antigens: A/New Caledonia, A/Panama, and B/Yamanashi or B/Victoria (B/Victoria was substituted for B/Yamanashi in the second year of data collection). Because previous literature has found psychological associations with changes in Ab levels with A viruses [21] and past work with this data set has found associations with only the A/New Caledonia virus [34], for the purposes of this study, only the A/New Caledonia virus was considered.
Ab titers were quantified using a standard hemagglutination inhibition protocol. To quantify the volume of a participant’s Ab level, his or her serum was diluted with various saline concentrations and then added to a red blood cell culture that contained influenza. The titer is the reciprocal of the highest dilution at which a person’s serum continues to prevent red cells from clumping. Thus, higher titer values indicate greater volumes of antibodies to the vaccine component. All samples were run in duplicate as well as a nonantigen control, and all time points for each participant were run in the same assay contemporaneously. The antigen used to check Ab levels for the A/New Caledonia virus was A/New Caledonia/20/99 with a hemagglutination titer of 1024 used at four hemagglutinating units (HAU)/25 μL. The A/New Caledonia was obtained from the World Health Organization collaborating center.
Statistical Analysis
The dependent variable in all analyses was Ab level at either one or four months post-vaccination. The independent variables of interest were NASD and PASD and their interactions with NAMEAN and PAMEAN, respectively. NAMEAN, PAMEAN, NASD, and PASD were all centered to allow for ease of their interpretations in interaction terms. An interaction term between NASD and NAMEAN allowed for a test of whether different values of variability in NA had differential implications for Ab response at different levels of mean NA. The same was true for the interaction between PASD and PAMEAN. Additionally, before interaction terms were added into the model, models with only the main effects were tested.
Ordinary least squares regression models [35] were used to investigate the effect of the independent variables on Ab level at one and four months post-vaccination. Due to problems of non-constant error variance that was likely due to substantial negative skewness of the outcome variable that could not be alleviated using transformations, robust standard errors were used in all ordinary least squares regression models. Individuals who started with baseline levels at maximum level (N = 5) were excluded from the analyses, as this would not allow us to see a change in Ab level.
Consistent with previous research (e.g., [34]), standard control variables were included in analyses: baseline Ab level (i.e., immediately before the vaccination occurred), study cohort, sex, and ethnicity (Caucasian = 1; other = 0).
A power analysis revealed that for a linear regression with 7 predictors (e.g., NASD, NAMEAN, NASD*NAMEAN, baseline Ab level, female, Caucasian, and cohort), 80 participants would be required to detect a medium to large effect size with 80% power and an alpha error probability of 0.05 [36].
Results
Descriptive Statistics
NAMEAN and PAMEAN were significantly different in average value (t(82) = 8.40, p < .001, 95% CI of the difference [0.54, 0.88]), while NASD and PASD averages were closer in value but still significantly different (t(82) = 2.33, p = .011, 95% CI of the difference [0.01, 0.08]; see Table 1). NAMEAN and PAMEAN were negatively correlated (r = −0.31, p = .004), but NASD and PASD were positively correlated (r = 0.50, p < .001; see Table 1). In other words, those with greater NA variability also experienced greater PA variability. Although NAMEAN was positively correlated with NASD (r = 0.63, p < .001), PAMEAN was not correlated with PASD (r = 0.16, p = .140).
Table 1.
Descriptive Statistics of Affect Mean and Standard Deviation Measures.
| Measure | Mean | SD | 2 | 3 | 4 |
|---|---|---|---|---|---|
| 1. NAMEAN | 0.81 | 0.43 | −0.31** (−0.50, −0.10) |
0.63** (0.48, 0.75) |
0.11 (−0.11, 0.32) |
| 2. PAMEAN | 1.52 | 0.51 | −0.31** (−0.49, −0.10) |
0.16 (−0.06, 0.37) |
|
| 3. NASD | 0.46 | .17 | 0.50** (0.32, 0.64) |
||
| 4. PASD | 0.50 | .16 |
Note. NA = Negative Affect; PA = Positive Affect; SD = Standard Deviation. The last three columns are the correlation matrix of the variables. 95% confidence intervals are in parentheses.
p < 0.01;
p < 0.05
Affect Variability and Antibody Response
NASD was a predictor of Ab level at one and four months post vaccination (see Tables 2 and 3; one month: b = −574.31, SE = 271.41, p = .038, 95% CI [−1,115.35, −33.27]; four months: b = −692.48, SE = 274.86, p = .014, 95% CI [−1,240.54, −144.42]) when placed in a model not controlling for NAMEAN. This indicated that individuals who were lower in NA variability were more likely to exhibit higher levels of Ab post-vaccination. However, after controlling for NAMEAN, NASD was not significantly associated with Ab level at one month (see Table 2; b = −485.84, SE = 341.59, p = .159, 95% CI [−1,166.96, 195.28]) and marginally associated with Ab level at four months post-vaccination (see Table 3; b = −660.30, SE = 374.67, p = .082, 95% CI [−1,407.55, 86.96]). Additionally, there was no significant interaction between NASD and NAMEAN (see Tables 2 and 3; one month: b = −201.65, SE = 681.71, p = .768, 95% CI [−1,561.28, 1,157.98]; four months: b = 64.43, SE = 696.26, p = .927, 95% CI [−1,324.58, 1,453.44]).
Table 2.
Standard Deviation and Mean of NA and PA Predicting Antibody Level at 1 Month Post-Vaccination.
| NA
|
PA
|
NA and PA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 | |
| NASD |
−574.31* (−1,115.35, −33.27) 271.41 |
−485.84 (−1,166.95, 195.28) 341.59 |
−460.24 (−1,181.29, 260.82) 361.53 |
−762.24† (−1,527.09, 2.62) 383.19 |
|||||
| NAMEAN | −164.19 (−367.84, 39.45) 102.16 |
−54.36 (−321.46, 212.74) 133.96 |
−68.03 (−337.04, 200.98) 134.88 |
−27.04 (−276.62, 222.54) 125.04 |
|||||
| NASD* NAMEAN |
−201.65 (−1,561.28, 1,157.98) 681.71 |
−204.02 (−1,307.20, 899.16) 552.69 |
|||||||
| PASD |
−456.76† (−996.70, 83.18) 270.85 |
−422.03 (−950.45, 106.39) 265.01 |
−453.83† (−906.75, −0.92) 227.09 |
4.80 (−660.25, 669.86) 333.19 |
|||||
| PAMEAN | −74.76 (−259.76, 110.24) 92.80 |
−52.56 (−228.7, 123.66) 88.38 |
−118.26 (−289.98, 53.45) 86.10 |
−223.91* (−399.12, −48.70) 87.78 |
|||||
| PASD* PAMEAN |
−2,070.98** (−3,277.37, −864.59) 604.88 |
−2,255.77** (−3,561.88, −949.67) 654.36 |
|||||||
| Baseline Ab Level |
0.32 (−1.51, 2.15) 0.92 |
0.42 (−1.43, 2.28) 0.93 |
0.29 (−1.59, 2.16) 0.94 |
0.34 (−1.61, 2.29) 0.98 |
0.64 (−1.02, 2.30) 0.83 |
0.86 (−0.68, 2.40) 0.77 |
0.76 (−0.76, 2.28) 0.76 |
0.84 (−0.46, 2.13) 0.65 |
0.63 (−0.85, 2.10) 0.74 |
| Female (1 = female) |
26.28 (−136.03, 188.58) 81.42 |
15.88 (−147.79, 179.54) 82.10 |
27.34 (−137.77, 192.44) 82.80 |
27.60 (−137.79, 193.00) 82.93 |
12.34 (−146.96, 171.65) 79.91 |
−9.39 (−169.63, 150.85) 80.38 |
4.28 (−153.33, 161.89) 79.05 |
43.98 (−107.45, 195.40) 75.92 |
58.64 (−96.97, 214.25) 77.96 |
| Caucasian (1 = Caucasian) |
163.04† (−12.62, 338.71) 88.12 |
146.68† (−26.76, 320.11) 87.00 |
163.67† (−13.31, 340.66) 88.76 |
158.17† (−29.68, 346.02) 94.18 |
149.42† (−23.76, 322.60) 86.87 |
127.07 (−47.43, 301.56) 87.53 |
146.59 (−29.68, 322.86) 88.40 |
88.26 (−80.44, 256.96) 84.58 |
102.64 (−64.23, 269.50) 83.60 |
| Cohort |
−334.34** (−523.50, −145.17) 94.89 |
−347.37** (−540.75, −153.99) 97.01 |
−336.17** (−526.99, −145.35) 95.70 |
−335.36** (−528.35, −142.37) 96.76 |
−328.58** (−524.23, −132.92) 98.15 |
−339.11** (−538.28, −139.93) 99.91 |
−323.58** (−521.84, −125.33) 99.43 |
−312.62** (−505.06, −120.19) 96.48 |
−299.16** (−461.92, −136.41) 81.54 |
| Constant | 749.47** | 802.09** | 757.46** | 769.29** | 771.96** | 800.79** | 772.91** | 807.53** | 777.89** |
Note. Robust 95% confidence intervals in parentheses; Robust standard errors below confidence intervals; NA = Negative Affect; PA = Positive Affect; SD = Standard Deviation; Ab = Antibody.
p < 0.01
p < 0.05
p < 0.10.
Table 3.
Standard Deviation and Mean of NA and PA Predicting Antibody Level at 4 Months Post-Vaccination.
| NA
|
PA
|
NA and PA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 | |
| NASD | −692.48* (−1,240.54, −144.42) 274.86 |
−660.30† (−1,407.55, 86.96) 374.67 |
−668.69† (−1,436.65, 99.28) 384.96 |
−909.15* (−1,803.80, −14.51) 448.09 |
|||||
| NAMEAN | −173.45 (−381.26, 34.35) 104.22 |
−20.14 (−321.67, 281.38) 151.18 |
−15.58 (−325.40, 294.25) 155.31 |
17.26 (−274.40, 308.91) 146.08 |
|||||
| NASD* NAMEAN |
64.43 (−1,324.58, 1,453.44) 696.26 |
71.52 (−1,063.81, 1,206.86) 568.64 |
|||||||
| PASD | −533.23† (−1,070.26, 3.79) 269.33 |
−515.75† (−1,048.80, 17.30) 267.27 |
−536.22* (−1,003.79, −68.64) 234.38 |
−26.88 (−681.64, 627.89) 327.95 |
|||||
| PAMEAN | −54.67 (−232.01, 122.67) 88.94 |
−29.75 (−197.40, 137.90) 84.06 |
−78.48 (−237.20, 80.24) 79.56 |
−191.49* (−343.80, −39.19) 76.28 |
|||||
| PASD* PAMEAN |
−1,653.02** (−2,666.66, −639.38) 508.10 |
−1,882.18** (−3,167.36, −597.01) 643.69 |
|||||||
| Baseline Ab Level |
1.22† (−0.13, 2.58) 0.68 |
1.39† (−0.00, 2.79) 0.70 |
1.21† (−0.17, 2.59) 0.69 |
1.19 (−0.25, 2.64) 0.72 |
1.60* (0.31, 2.89) 0.65 |
1.79** (0.46, 3.11) 0.67 |
1.66* (0.34, 2.99) 0.67 |
1.72* (0.40, 3.05) 0.66 |
1.44* (0.01, 2.87) 0.71 |
| Female (1 = female) |
−20.07 (−186.37, 146.23) 83.40 |
−36.75 (−206.29, 132.79) 85.03 |
−19.96 (−187.97, 148.04) 84.24 |
−20.00 (−189.30, 149.30) 84.86 |
−34.31 (−199.98, 131.37) 83.09 |
−55.73 (−222.35, 110.89) 83.56 |
−38.24 (−200.47, 123.98) 81.34 |
−8.12 (−169.26, 153.02) 80.77 |
6.46 (−155.95, 168.86) 81.34 |
| Caucasian (1 = Caucasian) |
120.24 (−59.23, 299.71) 90.01 |
100.75 (−78.64, 280.14) 89.97 |
120.99 (−59.30, 301.28) 90.40 |
122.65 (−71.10, 316.40) 97.12 |
98.43 (−78.78, 275.64) 88.87 |
73.89 (−110.07, 257.86) 92.26 |
96.04 (−87.39, 279.47) 91.97 |
52.33 (−133.17, 237.83) 92.98 |
76.06 (−112.06, 264.17) 94.22 |
| Cohort | −297.24** (−496.86, −97.61) 100.12 |
−315.96** (−521.00, −110.92) 102.83 |
−298.38** (−499.62, −97.13) 100.90 |
−298.54** (−501.93, −95.15) 101.95 |
−286.52** (−491.93, −81.12) 103.01 |
−303.61** (−513.96, −93.26) 105.49 |
−282.74** (−490.26, −75.22) 104.05 |
−277.59** (−483.26, −71.92) 103.10 |
−265.54** (−464.85, −66.22) 99.83 |
| Constant | 741.47** | 803.87** | 744.18** | 740.42** | 771.58** | 805.79** | 772.31** | 799.21** | 746.51** |
Note. Robust 95% confidence intervals in parentheses; Robust standard errors below confidence intervals; NA = Negative Affect; PA = Positive Affect; SD = Standard Deviation; Ab = Antibody.
p < 0.01
p < 0.05
p < 0.10.
PASD was marginally associated with Ab levels at one and four months post-vaccination (see Tables 2 and 3; one month: b = −456.76, SE = 270.85, p = .096, 95% CI [−996.70, 83.18]; four months: b = −533.23, SE = 269.33, p = .052, 95% CI [−1,070.26, 3.79]). When controlling for PAMEAN, this association became non-significant at one month (see Table 2; b = −422.03, SE = 265.01, p = .116, 95% CI [−950.45, 106.39]) and stayed marginally significant at four months post vaccination (see Table 3; b = −515.75, SE = 267.27, p = .058, 95% CI [−1,048.80, 17.30]). However, PASD did significantly interact with PAMEAN to predict Ab levels at both time points (see Tables 2 and 3; one month: b = −2,070.98, SE = 604.88, p = .001, 95% CI [−3,277.37, −864.59]; four months: b = −1,653.02, SE = 508.10, p = .002, 95% CI [−2,666.66, −639.38]). Specifically, at high PAMEAN, individuals with higher levels of PASD (i.e., more variability) were more likely to have lower Ab levels at follow-up (see Figures 2 and 3). This is evidenced by the negative slope of PASD at high PAMEAN (one month: dy/dx = −1,510.03, SE = 339.70, p < .001, 95% CI [−2,187.54, −832.52]; four months: dy/dx = −1,379.26, SE = 270.44, p < .001, 95% CI [−1,918.77, −839.75]). In contrast, when examining the simple effects of the slope of PASD at low PAMEAN, the slope was not significantly different from zero (one month: dy/dx = 602.36, SE = 421.98, p = .158, 95% CI [−239.26, 1,443.99]; four months: dy/dx = 306.82, SE = 413.56, p = .461, 95% CI [−518.20, 1,131.84]), indicating that PASD had no effect on Ab levels for individuals with lower PAMEAN.
Figure 2.
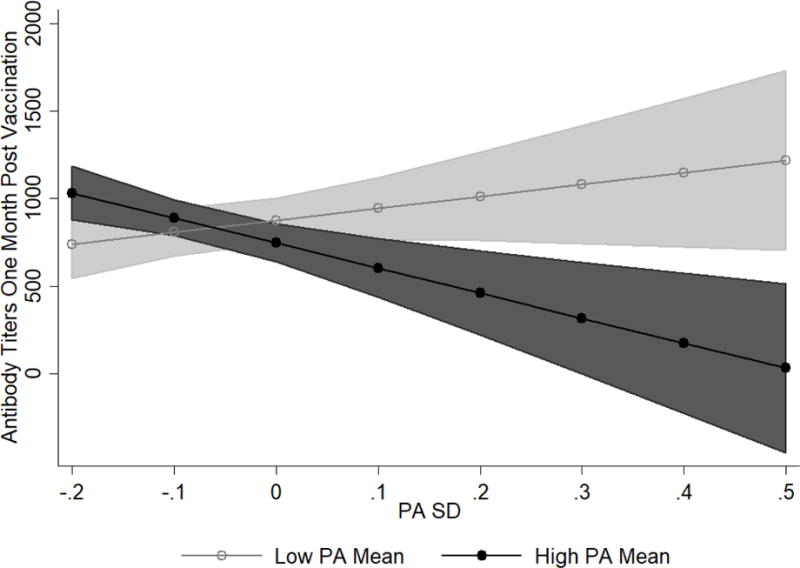
PASD and PAMEAN interaction on antibody (Ab) titers at one month post-vaccination. Low PAMEAN is one SD below the mean on PAMEAN while high PAMEAN is one SD above the mean on PAMEAN. Lines represent adjusted predictions. Shaded regions are the 95% confidence intervals around the predictions. Regions of PAMEAN (low vs. high) that do not overlap are significantly different from one another in terms of the predicted Ab level. Possible predicted values are between 0 and 1024 titers. However, confidence intervals exceed this range.
Figure 3.
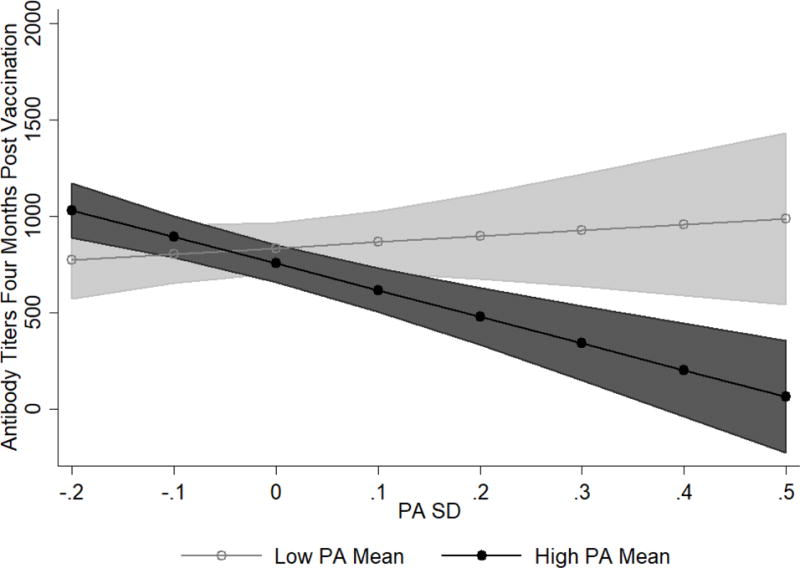
PASD and PAMEAN interaction on antibody (Ab) titers at four months post-vaccination. Low PAMEAN is one SD below the mean on PAMEAN while high PAMEAN is one SD above the mean on PAMEAN. Lines represent adjusted predictions. Shaded regions are the 95% confidence intervals around the predictions. Regions of PAMEAN (low vs. high) that do not overlap are significantly different from one another in terms of the predicted Ab level. Possible predicted values are between 0 and 1024 titers. However, confidence intervals exceed this range.
Interestingly, when the NA and PA variables were placed in the same model, the findings for the interaction between PASD and PAMEAN (see Tables 2 and 3; one month: b = −2,255.78, SE = 654.36, p = .001, 95% CI [−3,561.88, −949.67]; four months: b = −1,882.19, SE = 643.69, p = .005, 95% CI [−3,167.36, −597.01]) as well as the main effect of NASD (see Tables 2 and 3; one month: b = −762.24, SE = 383.19, p = .051, 95% CI [−1,527.09, 2.62]; four months: b = −909.15, SE = 448.09, p = .047, 95% CI [−1,803.80, −14.51] held2.
Discussion
Our findings indicate that affect variability, and the interaction between that variability and mean levels of affect, significantly influence immunocompetence in response to the flu vaccination. NA variability, along with the interaction between PAMEAN and PA variability, predicted Ab levels following a vaccination, and these findings held when they were all placed in the same model (i.e., model 9 in Tables 2 and 3). These results suggest that affect variability may have important implications for physical health, especially in the context of the immune system.
The results regarding PA are nuanced and depend on the interaction between PA mean and variability. Individuals high in PAMEAN who had low variability (consistently stayed at their high PA level as opposed to drastically bouncing up and down around it) had a robust immune response. However, if an individual had a high PAMEAN, high PA variability was detrimental for mounting a large Ab response. It is possible that for an individual with high PA variability, the negative ramifications of dropping far below a mean level are not offset by the peaks of being above a mean level. In contrast, for individuals with low levels of PAMEAN, variability in PA had no effects on Ab levels (see Figures 2 and 3). These PA findings are consistent with some of the current affect variability and physiological findings (e.g., [20] Study 1).
High NA variability was associated with lower Ab levels (see model 1 in Tables 2 and 3). However, when interpreting the meaning of these results, it is important to consider the connection between mean levels and variability. Standard deviations can be confounded with mean levels (e.g., [37]), and indeed, NAMEAN and NASD were highly correlated in this study (see Table 1). Therefore, it was important to control for NAMEAN when determining the effect of NASD on Ab levels. When controlling for NAMEAN, the main effect of NASD became nonsignificant at one month and marginal at four months post vaccination. This could imply that NAMEAN was an important factor driving the association between NASD and Ab levels. However, the coefficient of NASD did not dramatically reduce from model 1 without the mean level control (one month: −574.31; four months: −692.48) to model 3 with the mean level control (one month: −485.84; four months: −660.30), suggesting that maybe with more participants, this effect would have remained significant. Nevertheless, given the high correlation between mean level and standard deviation, we tested our models with the alternative variability indicator of the mean square successive differences (MSSD). We found no main effect of NAMSSD in any of the models. Interestingly, NAMEAN and NAMSSD were not significantly correlated (r = 0.16, p = 0.16), in contrast to the high correlation between NAMEAN and NASD (r = 0.63, p < .001). This may suggest that even when NA variability is not confounded with mean levels, NA variability has little effect on biomarkers (at least in healthy college samples). With regard to PA, PAMEAN and PASD were not confounded (see Table 1), which may explain why there were interactive effects of PAMEAN and PASD on Ab levels. It is interesting that NA variability is related to NAMEAN levels while PA variability is not related to PAMEAN levels. Indeed, many studies only find effects with PA variability as opposed to NA variability (e.g., [9,20]), providing evidence that these two valence types should be examined as separate variables as opposed to combining them into one variability variable.
Why is affect variability associated with Ab levels? One explanation may be connected to the influence of affect variability on affective processes. High affect variability may be indicative of poor affect regulation, which has implications for health [38, 39]. Additionally, affect variability is a key factor in neuroticism (e.g., [40]), and high levels of neuroticism have been shown to negatively influence immune response [41]. Alternatively, affect variability might be related to health through its influence on health behaviors. For example, it is possible that affect variability impacts sleep, eating behaviors, and/or the use of alcohol and tobacco (all of which have been shown to affect immunity [22, 42]). Yet another explanation could be that affect variability may alter the concentration of circulating stress-related hormones (e.g., cortisol, epinephrine, norepinephrine), which can bind to and alter the function of immune cells involved in the Ab response [43]. These possible pathways are likely interrelated and may all contribute in some way to how affect variability gets “under the skin” to influence Ab responses to vaccines. Thus, future research should consider these potential pathways in the affect variability and Ab response relationship to determine how affect variability influences immune responses.
This study has several potential limitations. First, although we used a common metric of affect variability, namely, the standard deviation approach, there are also other methods (e.g., insufficient variations, adjusted squared successive difference scores, core affect variability; [40, 44–47]) that were not considered. However, we chose the standard deviation approach since it is easy to understand and the most commonly used technique, in addition to the fact that different variability measures often lead to similar results (e.g., [9, 46], our results reported above with MSSD). A second limitation was that we did not include the effect of affect variability before the vaccination. Because past research has shown that psychological variables are more influential after the vaccine [42], we used only the days on or after the flu vaccination to calculate variability3. Third, we only considered one virus. This decision was based on previous findings that psychosocial variables influence responses to only some virus strains [24, 41]. Although it is difficult to say whether certain viruses are more sensitive to psychosocial factors, it has been suggested that there may be differences in strain novelty and the participant’s previous exposure to the strain that could explain the differing findings in the literature [48].
Fourth, statistical significance does not translate to clinical significance. In our sample, all but 5 participants at one month and all but 6 participants at four months post vaccination had Ab levels above 40 titers, which is considered the clinically protective level [49, 50]. Similar vaccine studies using young adult samples have shown that the majority of the sample are protected against the virus after vaccination (e.g., [34, 51]). Nevertheless, there was sufficient titer variability within our sample to see statistically significant associations with affect variability and mean levels. Additionally, we included pre-vaccine levels as a control variable in all analyses (as suggested by [49]). Therefore, our findings still speak to how affect variability may influence immune responses to vaccines in young, healthy individuals.
This study emphasizes the importance of assessing the effects of affect variability in addition to mean levels of affect on an objective health-relevant biomarker. Mean levels of affect do not explain the whole story about how an individual may mount an immune response. Previous research may have overlooked important intricacies about the influence of affect because variability was not assessed. In our study, if we only considered mean levels of PA or NA, then we would have found no influence of affect on Ab response. This would have resulted in an incomplete understanding of the effects of affect on vaccination response. The results of this study should encourage future researchers to consider affect variability in addition to mean levels of affect and point to the possibility that it may have unique effects on other physical health-relevant parameters. Assessing vacillations in affect will help paint a more vivid picture of how our affective experiences influence physical health.
Highlights.
Affect variability has important implications for immune response.
Negative affect variability is related to lower antibody production.
Positive affect variability and mean levels interact to influence antibody production.
Future researchers should consider how affect variability influences health.
Acknowledgments
We would like to thank Drs. John Treanor and Dominik Wodarz for their extensive and helpful feedback on previous versions of this manuscript. Brooke N. Jenkins was supported by the Ruth L. Kirschstein National Research Service Award (HD085712) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Sarah D. Pressman’s time was partially supported by an award from the AXA Research Fund. Data collection was supported by a grant from the National Institute of Mental Health (MH50430) and supplemental funding from the John D. and Catherine T. MacArthur Foundation Research Network on Socioeconomic Status and Health. Data collection was also facilitated by grants from the National, Heart, Lung, and Blood Institute to the Pittsburgh Mind-Body Center (HL65111 and HL65112).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We used only these days because past research has shown that psychological variables are more influential after the vaccine [42].
Past studies have looked at other variables including loneliness, social network, and stress related to vaccine response (including studies that have used this data set). When considering these psychosocial variables, the pattern of results, especially the interaction terms of interest, was unaffected. The only exceptions to this were that PASD became significant as a main effect and NASD became non-significant in some models. This non-significance may reflect the substantial overlap in NASD with loneliness (r = 0.47, p < .001) and stress (r = 0.52, p < .001).
Furthermore, we felt that the two days before the vaccination would not provide sufficient data because participants were still new to self-reporting and that two days might not be sufficient to capture a large enough sample to reflect variability before the vaccination. Therefore, the first two days were considered to be a diary run-in.
References
- 1.Chida Y, Steptoe A. Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosom Med. 2008;70:741–56. doi: 10.1097/PSY.0b013e31818105ba. [DOI] [PubMed] [Google Scholar]
- 2.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–71. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 3.Prather AA, Marsland AL, Muldoon MF, Manuck SB. Positive affective style covaries with stimulated IL-6 and IL-10 production in a middle-aged community sample. Brain Behav Immun. 2007;21:1033–1037. doi: 10.1016/j.bbi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: The problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 5.Larsen RJ, Diener E. A multitrait-multimethod examination of affect structure: Hedonic level and emotional intensity. Pers Individ Dif. 1985;6:631–636. doi: 10.1016/0191-8869(85)90013-3. [DOI] [Google Scholar]
- 6.Pace TWW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, Raison CL. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci U S A. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houben M, Van Den Noortgate W, Kuppens P. The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychol Bull. 2015;141:901–930. doi: 10.1037/a0038822. [DOI] [PubMed] [Google Scholar]
- 9.Gruber J, Kogan A, Quoidbach J, Mauss IB. Happiness is best kept stable: Positive emotion variability is associated with poorer psychological health. Emotion. 2013;13:1–6. doi: 10.1037/a0030262. [DOI] [PubMed] [Google Scholar]
- 10.Hardy J, Segerstrom SC. Intra-individual variability and psychological flexibility: Affect and health in a National US sample. J Res Pers. 2016 doi: 10.1016/j.jrp.2016.04.002. [DOI] [Google Scholar]
- 11.Boehm JK, Winning A, Segerstrom S, Kubzansky LD. Variability modifies life satisfaction’s association with mortality risk in older adults. Psychol Sci. 2015;26:1063–1070. doi: 10.1177/0956797615581491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eizenman DR, Nesselroade JR, Featherman DL, Rowe JW. Intraindividual variability in perceived control in a older sample: The MacArthur successful aging studies. Psychol Aging. 1997;12:489–502. doi: 10.1037/0882-7974.12.3.489. [DOI] [PubMed] [Google Scholar]
- 13.Gadermann A, Zumbo B. Investigating the intra-individual variability and trajectories of subjective well-being. Soc Indic Res. 2007;81:1–33. [Google Scholar]
- 14.Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune responses: A meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- 15.Futterman A, Kemeny M, Shapiro D, Fahey J. Immunologic and physiological changes associated with induced positive and negative mood. Psychosom Med. 1994;56:499–511. doi: 10.1097/00006842-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Shiota MN, Neufeld SL, Yeung WH, Moser SE, Perea EF. Feeling good: Autonomic nervous system responding in five positive emotions. Emotion. 2011;11:1368–1378. doi: 10.1037/a0024278. [DOI] [PubMed] [Google Scholar]
- 17.Stone A, Cox D, Valdimarsdottir H, Jandorf L, Neale J. Evidence that secretory IgA antibody is associated with daily mood. J Pers Soc Psychol. 1987;52:988. doi: 10.1037//0022-3514.52.5.988. [DOI] [PubMed] [Google Scholar]
- 18.Ganzel BL, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychol Rev. 2010;117:134–174. doi: 10.1037/a0017773.Allostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwen BS, Seeman T. Stress and affect: Applicability of the concepts of allostasis and allostatic load, in. Handb Affect Sci. 2003:1117–1137. [Google Scholar]
- 20.Human LJ, Whillans AV, Hoppmann CA, Klumb P, Dickerson SS, Dunn EW. Finding the middle ground: Curvilinear associations between positive affect variability and daily cortisol profiles. Emotion. 2015;15:705–720. doi: 10.1037/emo0000071. [DOI] [PubMed] [Google Scholar]
- 21.Burns VE, Carroll D, Drayson M, Whitham M, Ring C. Life events, perceived stress and antibody response to influenza vaccination in young, healthy adults. J Psychosom Res. 2003;55:569–572. doi: 10.1016/S0022-3999(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S, Miller GE, Rabin BS. Psychological stress and antibody response to immunization: A critical review of the human literature. Psychol Med. 2001;63:7–18. doi: 10.1097/00006842-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Vedhara K, Fox JD, Wang ECY. The measurement of stress-related immune dysfunction in psychoneuroimmunology. Neurosci Biobehav Rev. 1999;23:699–715. doi: 10.1016/S0149-7634(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 24.Phillips AC, Carroll D, Burns VE, Ring C, Macleod J, Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain Behav Immun. 2006;20:279–289. doi: 10.1016/j.bbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Ong AD, Ram N. Fragile and enduring positive affect: Implications for adaptive aging. Gerontology. 2017;63:263–269. doi: 10.1159/000453357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diener E, Emmons R, Larsen R, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 27.Eid M, Diener E. Intraindividual variability in affect: Reliability, validity, and personality correlates. J Pers Soc Psychol. 1999;76:662–676. doi: 10.1037/0022-3514.76.4.662. [DOI] [Google Scholar]
- 28.Ram N, Gerstorf D. Time-structured and net intraindividual variability: Tools for examining the development of dynamic characteristics and processes. Psychol Aging. 2009;24:778–91. doi: 10.1037/a0017915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Röcke C, Brose A. Intraindividual variability and stability of affect and well-being. GeroPsych (Bern) 2013;26:185–199. doi: 10.1024/1662-9647/a000094. [DOI] [Google Scholar]
- 30.Usala PD, Hertzog C. Measurement of affective states in adults. Res Aging. 1989;11:403–426. doi: 10.1177/0164027589114001. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Emotional style and susceptibility to the common cold. Psychosom Med. 2003;65:652–657. doi: 10.1097/01.PSY.0000077508.57784.DA. [DOI] [PubMed] [Google Scholar]
- 32.Larson R. Adolescents’ daily experience with family and friends: Contrasting opportunity systems. J Marriage Fam. 1983;45:739–750. [Google Scholar]
- 33.Penner LA, Shiffman S, Paty JA, Fritzsche BA. Individual differences in intraperson variability in mood. J Pers Soc Psychol. 1994;66:712–721. doi: 10.1037/0022-3514.66.4.712. [DOI] [PubMed] [Google Scholar]
- 34.Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. 2005;24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- 35.StataCorp. Stata Statistical Software: Release 14. 2015 [Google Scholar]
- 36.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 37.Baird BM, Le K, Lucas RE. On the nature of intraindividual personality variability: Reliability, validity, and associations with well-being. J Pers Soc Psychol. 2006;90:512–527. doi: 10.1037/0022-3514.90.3.512. [DOI] [PubMed] [Google Scholar]
- 38.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 39.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. doi: 10.1037//0033-2909.98.2.310. [DOI] [PubMed] [Google Scholar]
- 40.Kuppens P, Van Mechelen I, Nezlek JB, Dossche D, Timmermans T. Individual differences in core affect variability and their relationship to personality and psychological adjustment. Emotion. 2007;7:262–274. doi: 10.1037/1528-3542.7.2.262. [DOI] [PubMed] [Google Scholar]
- 41.Phillips AC, Carroll D, Burns VE, Drayson M. Neuroticism, cortisol reactivity, and antibody response to vaccination. Psychophysiology. 2005;42:232–238. doi: 10.1111/j.1469-8986.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 42.Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS, Treanor JJ. Psychological stress and antibody response to influenza vaccination: When is the critical period for stress, and how does it get inside the body? Psychosom Med. 2004;66:215–223. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- 43.Rabin BS. Stress, immune function, and health: The connection. Wiley-Liss; New York: 1999. [Google Scholar]
- 44.Eaton LG, Funder DC. Emotional experience in daily life: Valence, variability, and rate of change. Emotion. 2001;1:413–421. doi: 10.1037/1528-3542.1.4.413. [DOI] [PubMed] [Google Scholar]
- 45.Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychol Methods. 2008;13:354–375. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- 46.Röcke C, Li SC, Smith J. Intraindividual variability in positive and negative affect over 45 days: Do older adults fluctuate less than young adults? Psychol Aging. 2009;24:863–878. doi: 10.1037/a0016276. [DOI] [PubMed] [Google Scholar]
- 47.Trull TJ, Solhan MB, Tragesser SL, Jahng S, Wood PK, Piasecki TM, Watson D. Affective instability: Measuring a core feature of borderline personality disorder with ecological momentary assessment. J Abnorm Psychol. 2008;117:647–661. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- 48.Vedhara K, Cox NK, Wilcock GK, Perks P, Hunt M, Anderson S, Lightman SL, Shanks NM. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999;353:627–631. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- 49.Beyer WEP, Palache AM, Lüchters G, Nauta J, Osterhaus ADME. Seroprotection rate, mean fold increase, seroconversion rate: Which parameter adequately expresses seroresponse to influenza vaccination? Virus Res. 2004;103:125–132. doi: 10.1016/j.virusres.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Cox J, DeSouza M, Ratto-Kim S, Ferrari G, Weinhold K, Birx D. Cellular immune assays for evaluation of vaccine efficacy in developing countries. In: Rose N, Hamilton R, Detrick B, editors. Man Clin Lab Immunol. ASM Press; Washington, DC: 2002. pp. 301–317. [Google Scholar]
- 51.Glaser R, Kiecolt-Glaser J, Bonneau R, Malarkey W, Kennedy S, Hughes J. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med. 1992;54:22–29. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]


