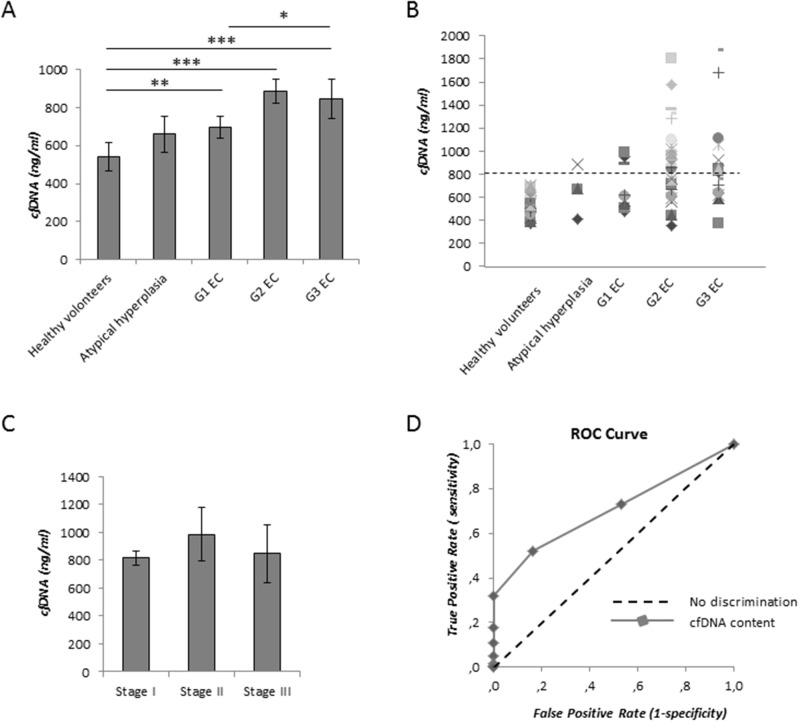
Figure 1. Measurement of cfDNA content with SYBR gold assay in EC patients.
(A) Comparison among cfDNA levesl in serum samples of healthy volunteers (n=21), of patients with atypical hyperplasia (n=6), of G1 EC patients (n=12), G2 EC (n=28), G3 EC (n=17). (B) CfDNA distribution in our cohort of serum samples. Dashed line indicates the cutoff value for cfDNA (800 ng/ml). (C) Average of cfDNA release in serum samples according to EC stage. Statistical significance: *P≤0.05. Data are represented as mean +/− standard error (SEM). The error bars indicate the standard error. (D) Receiver operative characteristics (ROC) curves for cfDNA levels measured with SYBR gold stain resulting by comparing cfDNA levels from healthy volunteers and EC patients.