Figure 2.
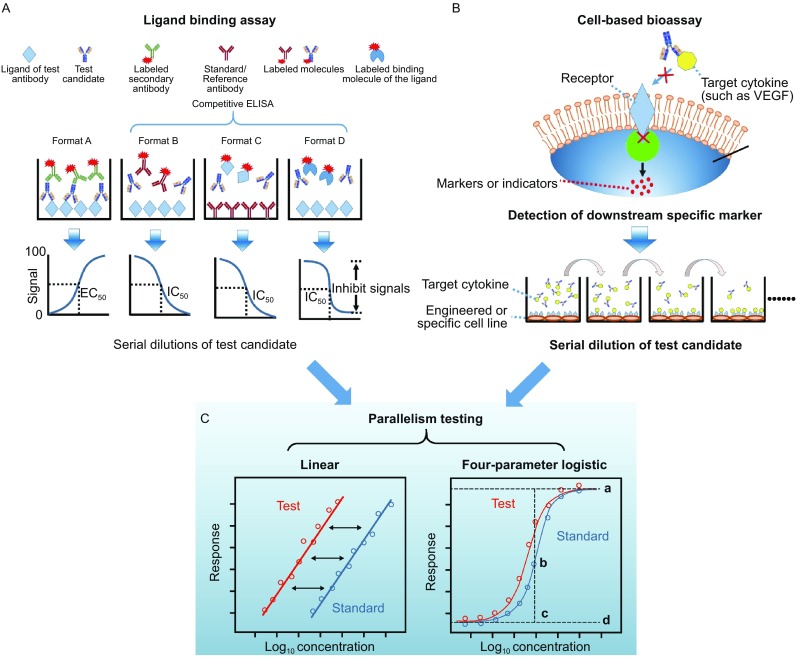
Schematic diagrams of ligand-binding assays and cell-based potency assay. (A) Four different types of ELISA-based ligand-binding assays (Biacore assays can be designed in a similar way). Format A is a direct way to evaluate the binding activity to the intended receptor. Formats B and C are both competitive ELISA; Format C is highly preferred for purified IgG since there are no subsequent wash cycles for the test antibody, unlike in the other three types. Format D is a ligand-blocking assay in the form of a competitive ELISA. (B) Cell based bioassay. The therapeutic mAbs are generally target cytokines or cell-surface receptors (In this diagram, the mAbs target cytokines as an example). Based on the understanding of the mechanism of action of mAbs, an engineered or specific cell line should be developed, and the marker should be defined. For example, human umbilical vein endothelial cells or the NFAT-RE-luc2P/KDR HEK293 cell line are used to assess the mAbs of VEGF, and the calcineurin-NFAT pathway could be used as the key marker of VEGF-mediated angiogenesis. (C) Parallelism test between data sets for test articles and the reference. Data obtained from the ligand binding assays and cell-based assay was recommended to analysis by using parallelism tests, including linear model and four-parameter logistic model, to estimating sample potency relative to a given standard. The symbols “a” in four-parameter logistic model represent upper asymptote, “b” represent slope parameter, “c” represent EC50, “d” represent lower asymptote
