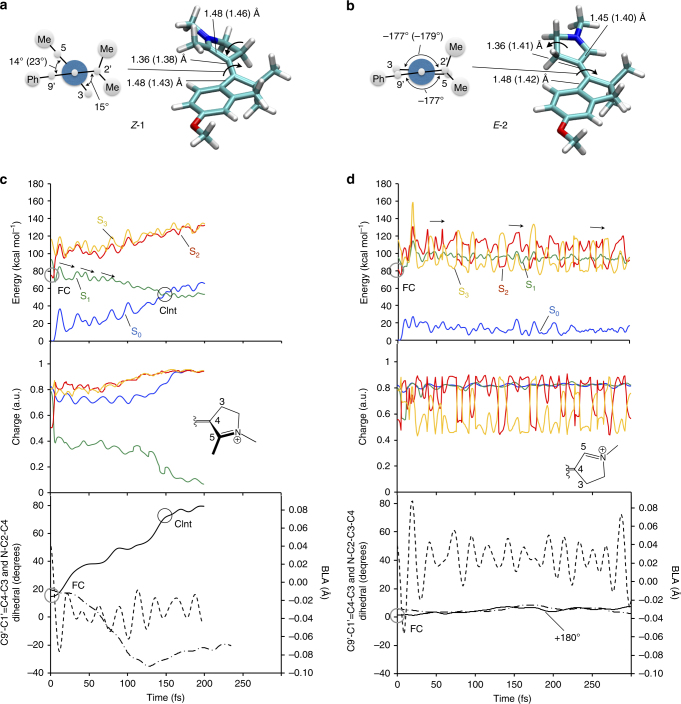
Fig. 4.
Mechanistic interpretation of the influence of the methyl substitution on C5. a, b Structure of the computed S0 free energy minima of Z-1 and E-2 in methanol at room temperature and values of their relevant structural parameters. The values for the corresponding S1 energy minima are given in parenthesis and the values of the C9ʹ–C1ʹ=C4–C3 and C2ʹ–C1ʹ=C4–C3 torsional parameters are given in the Newman projections on the left. A comparison with the available observed crystallographic parameters is given in Supplementary Fig. 2. c, d Computed S1 trajectories of Z-1 and E-2, respectively, in methanol solution, illustrated by (top panels) the S0, S1, and S2 CASPT2//CASSCF/6-31G*/Amber energy profiles, (middle panels) the changes in electronic structure along the trajectories in terms of the fractional positive charge remaining on the pyrroline moiety of the switch, and (bottom panels) the progression along the reaction coordinate described through skeletal double-bond stretching (BLA, dashed line), out-of-plane deformation of the pyrroline ring (reflected by the =N–C2–C3–C4- dihedral, dashed-dotted line), double-bond twisting (C9ʹ–C1′=C4–C3, full line). FC indicates the configurations of a and b. CInt indicates a conical intersection point. Notice the slow progression of E-2 along both twisting and out-of-plane deformation coordinates