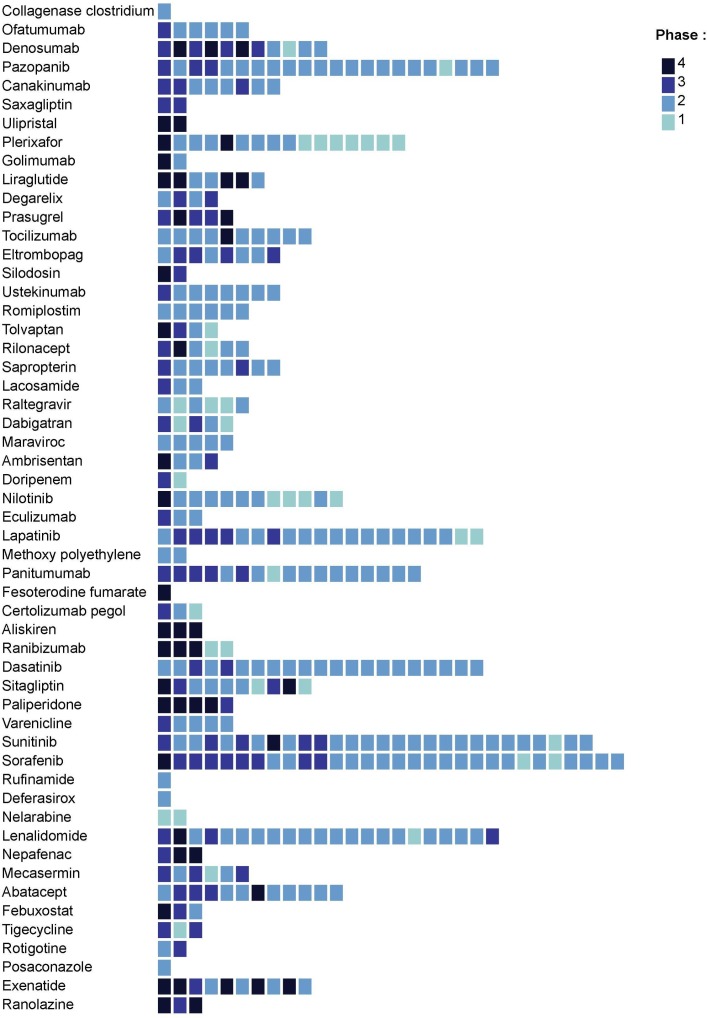
Figure 2.
Number of non-approved indications targeted in postmarketing studies for each drug of our study sample. Indications are rank-ordered on the basis of the number of postmarketing studies launched (from the greatest number of postmarketing studies on the left side of the figure to the lowest number on the right side). Colour of boxes varies according to the advanced phase of the targeted indication. Indications are classified according to the Global Burden of Diseases classification.21 Indications belonging to residual categories or health conditions not relevant to the Global Burden of Diseases were excluded and therefore are not represented in the figure.