Abstract
Mammals with a carotid rete are capable of reducing brain temperature below that of carotid blood temperature, termed selective brain cooling. In artiodactyls, selective brain cooling conserves body water and may provide them with a selective advantage in conditions of increased temperature and aridity.
Keywords: Artiodactyl success, brain temperature, carotid arterial blood temperature, osmoregulation, physiological plasticity, rostral epidural rete mirabile
Abstract
Some mammals have the ability to lower their hypothalamic temperature below that of carotid arterial blood temperature, a process termed selective brain cooling. Although the requisite anatomical structure that facilitates this physiological process, the carotid rete, is present in members of the Cetartiodactyla, Felidae and Canidae, the carotid rete is particularly well developed in the artiodactyls, e.g. antelopes, cattle, sheep and goats. First described in the domestic cat, the seemingly obvious function initially attributed to selective brain cooling was that of protecting the brain from thermal damage. However, hyperthermia is not a prerequisite for selective brain cooling, and selective brain cooling can be exhibited at all times of the day, even when carotid arterial blood temperature is relatively low. More recently, it has been shown that selective brain cooling functions primarily as a water-conservation mechanism, allowing artiodactyls to save more than half of their daily water requirements. Here, we argue that the evolutionary success of the artiodactyls may, in part, be attributed to the evolution of the carotid rete and the resulting ability to conserve body water during past environmental conditions, and we suggest that this group of mammals may therefore have a selective advantage in the hotter and drier conditions associated with current anthropogenic climate change. A better understanding of how selective brain cooling provides physiological plasticity to mammals in changing environments will improve our ability to predict their responses and to implement appropriate conservation measures.
Introduction
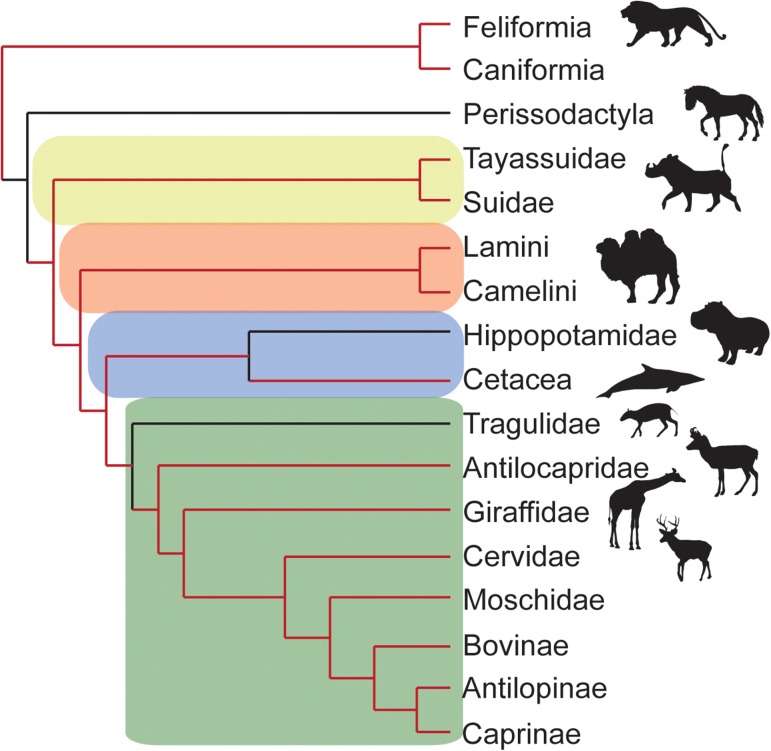
The carotid rete, or rostral epidural rete mirabile, is an intracranial vascular structure, near-ubiquitous and often elaborate in the Ruminantiamorpha, Whippomorpha, Camelidamorpha and Suinamorpha, collectively known as the Cetartiodactyla (Fig. 1; nomenclature sensu Spaulding et al. (2009)). The carotid rete is present also, often in a rudimentary or primitive form, in a number of laurasiatherian mammals (Ask-Upmark, 1935; du Boulay and Verity, 1973), including cats (Kamijyo and Garcia, 1975), in which it is extracranial (Daniel et al., 1953), and domestic dogs (Canis lupus familiaris; Daniel et al., 1953; Gillilan, 1976). Primates, many small-mass mammals (for example, rodents, lagomorphs and ‘insectivores’) and perissodactyls (horses, tapirs and rhinoceroses), a sister group of the artiodactyls (Hassanin et al., 2012), have no carotid rete (Ask-Upmark, 1935; Sisson and Grossman, 1967; Gillilan, 1974). In artiodactyls (e.g. antelopes, cattle, sheep and goats), anatomical investigations (Ask-Upmark, 1935; Daniel et al., 1953; Gillilan, 1974; Carlton and McKean, 1977; Frąckowiak et al., 2015; Kiełtyka-Kurc et al., 2015), including the identification of osteological correlates in extant and extinct artiodactyls (O'Brien, 2016), and physiological studies (Johnsen et al., 1987; Mitchell et al., 1997; Fuller et al., 1999b; Maloney et al., 2002; Lust et al., 2007; Hebert et al., 2008; Hetem et al., 2012; Strauss et al., 2016) have confirmed the presence of the rete and its functionality in virtually all of the extant terrestrial artiodactyls, with informative exceptions (Fig. 1). In the vast majority of terrestrial artiodactyls, the carotid rete is found in lieu of the internal carotid artery and serves as the main supply of oxygenated blood to the brain (Schummer et al., 1981; Wible, 1984; Frąckowiak, 2006; O'Leary, 2010; O'Brien, 2015). An exception is the Tragulidae, which consist of three genera of small, forest-dwelling antelope that have retained an internal carotid artery instead of a carotid rete (Fukuta et al., 2007; O'Brien, 2015). Whether their unique cranial vasculature is a plesiomorphic (sensu Janis, 1984) or apomorphic characteristic (e.g. Clauss and Rössner, 2014) has not been resolved. The Hippopotamidae also are artiodactyls, and a historic paper identifies hippopotamuses as having a carotid rete (Chapman, 1881), but it is indistinct (du Boulay and Verity, 1973; O'Brien, 2016). The status of the rete in Hippopotamidae requires further investigation using modern techniques, because it was identified before the anatomical techniques of resin injection and maceration, for exploring vascular systems, became available.
Figure 1:
Phylogenetic tree indicating the relationship between the Cetartiodactyla, Perissodactyla and the Carnivora, represented by the cats and dogs (adapted from Hassanin et al., 2012). Red branches indicate clades with a carotid rete, capable of selective brain cooling. Black branches designate the absence of a carotid rete (Hippopotamidae largely data deficient). Also depicted in various shades are the Ruminantiamorpha (green), Whippomorpha (blue), Camelidamorpha (orange) and Suinamorpha (yellow).
The function of the carotid rete in the Cetacea is unknown, but in the terrestrial artiodactyls, cats and dogs, it is a heat exchanger that can be used to cool the brain, but importantly, the hypothalamic region, below the temperature of carotid arterial blood. This phenomenon, known as ‘selective brain cooling’, first was described in a domestic cat (Felis catus) almost 50 years ago (Baker and Hayward, 1967). The brain, as a metabolically active organ, usually has a temperature higher than that of the arterial blood perfusing it. The heat produced by the brain is removed by that blood, meaning that in the absence of a carotid rete, the brain is ~0.5°C warmer than arterial blood leaving the heart (Fuller et al., 2000; Maloney et al., 2009). The carotid rete, carrying arterial blood destined for the brain, is surrounded by venous blood in either a cavernous sinus (artiodactyls) or a pterygoid sinus (felids; Daniel et al., 1953). The venous blood is derived from the maxilloturbinates and other mucous surfaces of the mouth and nose, where it is cooled to well below arterial blood temperature by evaporation of water into inspired air, whether or not the mammal is panting (Kuhnen and Jessen, 1991). The thin walls and large surface area of the carotid rete vessels allow for efficient heat exchange between the arterial and venous blood, the result being that the arterial blood exiting the carotid rete into the brain and, subsequently, the hypothalamic tissue in that region, can be more than 1°C cooler than arterial blood entering the rete (Maloney et al., 2007).
Soon after the heat-exchange function of the carotid rete was discovered, investigations of selective brain cooling in domesticated and habituated wild mammals in captivity led to the conclusion that selective brain cooling served to protect the brain from reaching dangerously high temperatures (Baker and Hayward, 1967, 1968; Magilton and Swift, 1968; Baker, 1972, 1979; Taylor and Lyman, 1972; Baker and Chapman, 1977; Mitchell et al., 1987). That conclusion was influenced heavily by one measurement of selective brain cooling. Taylor and Lyman (1972) reported that brain temperature after induced exercise in habituated Thomson's gazelle (Gazella thomsonii) was as much as 2.7°C lower than carotid arterial blood temperature, a magnitude of selective brain cooling never seen before or since by anyone else, in any mammal. A protectionist function fitted well with the perceived vulnerability of brain tissue to thermal damage in vitro (Burger and Fuhrman, 1964). Only when it was shown that goats (Capra hircus) could withstand brain temperatures of 42.5°C for an hour without any apparent ill effect (Caputa et al., 1986b) did it become apparent that the brain was not as vulnerable to thermal damage as believed previously (Mitchell et al., 2002). Indeed, rather than the brain, it is the tissue of the gastrointestinal tract that is most susceptible to thermal damage (Braasch, 1964), attributable to reduced splanchnic blood flow and endotoxin leakage (Leon and Helwig, 2010). Subsequent studies of free-living and unrestrained mammals have revealed that selective brain cooling is not obligatory at high body temperatures (Mitchell et al., 2002) and, as first noted in a laboratory study on goats, it is part of the normothermic thermoregulatory repertoire of artiodactyls (Kuhnen and Jessen, 1991). Rather than functioning primarily to protect the brain from thermal damage, selective brain cooling modulates the use of body water for thermoregulation (Jessen et al., 1998). By reducing the temperature of the hypothalamus, where the temperature sensors that provide the internal drive for heat loss are located, selective brain cooling reduces evaporative water loss (Kuhnen, 1997; Strauss et al., 2015). In the context of this alternative role for selective brain cooling, now described in textbooks of animal physiology (for example, Willmer et al., 2009; Withers et al., 2016), we provide here a perspective on the evolutionary and functional significance of selective brain cooling and its potential to provide physiological plasticity for artiodactyls facing hotter and drier environments associated with current climate change.
Factors controlling selective brain cooling
Control mechanisms govern the onset and degree of selective brain cooling in mammals with a carotid rete. The finding that selective brain cooling typically is exhibited by tame or habituated mammals when exposed to heat or exercise indicated that a primary input to the control of selective brain cooling is the mammal's internal temperature (Jessen et al., 1998). However, the absence of selective brain cooling in hyperthermic artiodactyls, particularly in free-living mammals during intense exercise, revealed that there also are non-thermal inputs in the control of selective brain cooling, and that these inputs can override thermal inputs (Mitchell et al., 2002; Fuller et al., 2014).
Thermal inputs
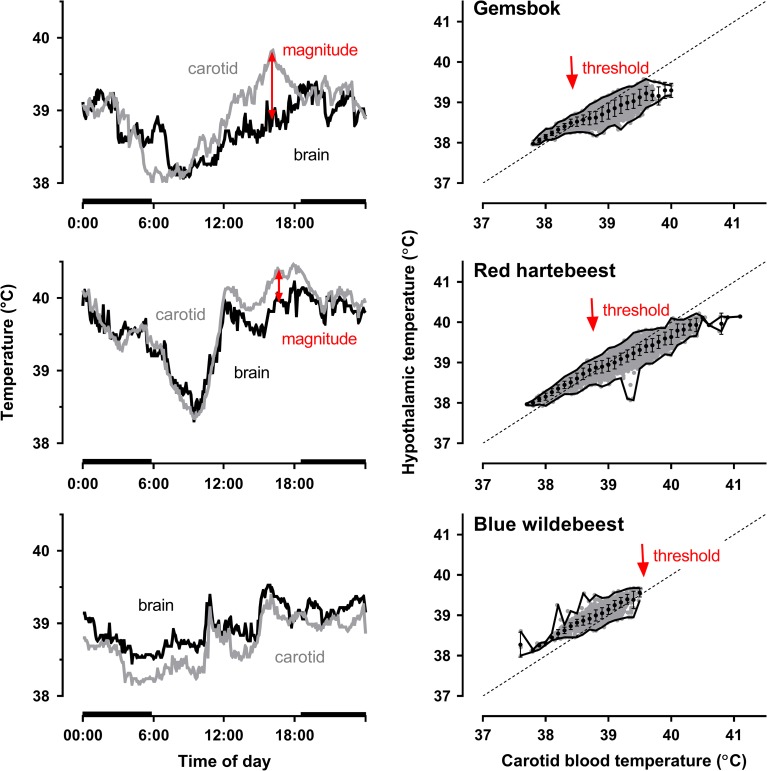
The typical relationship between hypothalamic temperature and pre-rete carotid arterial blood temperature in artiodactyls with carotid retes is shown in Fig. 2 (right panels). At the lower carotid arterial blood temperatures, brain temperature is higher than, and runs in parallel to, carotid arterial blood temperature, as illustrated for one gemsbok (Oryx gazella, upper panel), one red hartebeest (Alcelaphus buselaphus, middle panel) and one blue wildebeest (Connochaetes taurinus, lower panel) over the same 5 day period. In that temperature regime, in all mammals, whether or not they have a carotid rete, hypothalamic temperature generally exceeds carotid arterial blood temperature by about 0.2–0.5°C (Maloney et al., 2007). As carotid arterial blood temperature increases, hypothalamic temperature uncouples from carotid arterial blood temperature, as selective brain cooling ensues in those species with a carotid rete. Figure 2 also illustrates two variables that can be used to characterize selective brain cooling (red arrows): the temperature at which hypothalamic temperature and carotid arterial blood temperature are equal, which is the threshold temperature for selective brain cooling (Kuhnen and Jessen, 1991), and the extent to which brain temperature drops below carotid arterial blood temperature, the magnitude of selective brain cooling. The threshold temperature for selective brain cooling can differ, not only between species simultaneously exposed to the same environmental conditions (Fig. 2), but also both between individuals within a species (Strauss et al., 2016) and within an individual when exposed to different environmental conditions (Hetem et al., 2012), with the threshold temperature for selective brain cooling being reduced under high heat loads (Strauss et al., 2016).
Figure 2:
Left panels show the 24 h carotid blood and hypothalamic temperature profiles of a single gemsbok Oryx gazella (upper), red hartebeest Alcelaphus buselaphus (middle) and blue wildebeest Connochaetes taurinus (lower), for a single day, when the animals were free living in the same conditions in the Northern Cape Province, South Africa. Red arrows represent the magnitude of selective brain cooling within the 24 h period. Horizontal black bars indicate night time. Right panels show the correlation of hypothalamic temperature against carotid arterial blood temperature (grey circles) as well as hypothalamic temperature (mean ± SD) for every 0.1°C bin of simultaneous carotid arterial blood temperature, in the same gemsbok (upper), red hartebeest (middle) and blue wildebeest (lower) over a 5 day period during which they were exposed to the same environmental conditions. The boundary lines demonstrate the minimum and maximum hypothalamic temperatures in each bin. The diagonal line is the line of identity. Red arrows indicate the respective observed threshold temperatures for selective brain cooling; in the blue wildebeest the threshold was not reached within the range of measurement. Data from Strauss et al. (2016).
The finding that hyperthermia is a not a prerequisite for selective brain cooling is confirmed by measurements of the threshold temperature in many species (Table 1); it typically lies between 38 and 39°C (Table 1), close to the modal and mean body core temperature of artiodactyls (Hetem et al., 2016). In captive mammals, the magnitude of selective brain cooling typically increases with increasing carotid arterial blood temperature, but in free-living wild mammals it is more variable. Figure 2 (left panels) illustrates the carotid arterial blood and hypothalamic temperatures of three individual artiodactyls, a gemsbok (upper panel), a red hartebeest (middle panel) and a blue wildebeest (lower panel), living free in the same habitat and measured over the same 24 h period (Strauss et al., 2016). Like other large mammals (Mitchell et al., 2002; Hetem et al., 2016), these antelope had a 24 h rhythm of arterial blood temperature, with a trough soon after dawn and a peak in the late afternoon (Fig. 2, grey line). Hypothalamic temperature (Fig. 2, black line), which is determined mainly by post-carotid rete arterial blood temperature (Hayward et al., 1966), had a 24 h pattern similar to that of carotid arterial blood temperature, and the individuals from the three species exhibited a pattern of selective brain cooling similar to that typically exhibited by other free-living artiodactyls (Jessen et al., 1994; Mitchell et al., 1997; Hetem et al., 2012; Strauss et al., 2016). As carotid arterial blood temperature approached its 24 h peak, hypothalamic temperature uncoupled from carotid arterial blood temperature, and selective brain cooling ensued. Although selective brain cooling could be implemented at any time of the day (e.g. Fig. 2, middle left panel), its magnitude typically was greatest (0.5–1.5°C; Table 1) around the time of the 24 h peak in carotid arterial blood temperature. At that time of day, environmental heat load was decreasing, and the antelope usually were involved in low-intensity activities, such as rumination and grazing. Despite strong thermal inputs at that time of day, however, selective brain cooling in artiodactyls can be modulated by non-thermal inputs, such that its magnitude can be further increased or it can even be completely abolished.
Table 1:
The threshold temperature (mean ± SD, where originally reported) and maximum magnitude of selective brain cooling reported (or inferred) from studies of selective brain cooling in artiodactyls
| Species (sample size, n) | Selective brain cooling | Notes | Reference | |
|---|---|---|---|---|
| Threshold (°C) | Magnitude (°C) | |||
| Domestic or habituated animals studied in controlled laboratory conditions, unless otherwise indicated | ||||
| Goat Capra hircus (6) | Not reported | 2.5 | Heat exchanger | (Caputa et al., 1986a) |
| Goat Capra hircus (3) | 38.8 ± 0.1 | 1.2 | Heat exchanger | (Kuhnen and Jessen, 1991) |
| Goat Capra hircus (3) | 39.1 ± 0.1 | 0.5 | Heat exchanger and high humidity | (Kuhnen and Jessen, 1992) |
| Goat Capra hircus (3) | 39.2 ± 0.1 | 1.2 | Heat exchanger and low humidity | (Kuhnen and Jessen, 1992) |
| Goat Capra hircus (3) | 38.9 | 1.5 | Heat exchanger | (Kuhnen, 1997) |
| Goat Capra hircus (3) | 39.0 | 0.3 | Free-living, euhydration | (Jessen et al., 1998) |
| Goat Capra hircus (3) | 38.9 | 0.8 | Free-living, dehydration | (Jessen et al., 1998) |
| Goat Capra hircus (5) | 39.3 ± 0.1 | 0.7 | Hydrated and exercise | (Baker and Nijland, 1993) |
| Goat Capra hircus (5) | 39.3 | 1.2 | Dehydrated and exercise | (Baker and Nijland, 1993) |
| Ox Bos taurus (11) | 39.1 | 0.8 | Heat exposure | (Chesy et al., 1983) |
| Ox Bos taurus (3) | 40.3 | 1.5 | Exercise | (Chesy et al., 1985) |
| Sheep Ovis aries (3) | Not reported | 0.6 | Heat exchanger and heat exposure | (Maloney and Mitchell, 1997) |
| Sheep Ovis aries (4) | Not reported | 1.0 | Room temperature | (Laburn et al., 1988) |
| Sheep Ovis aries (4) | Not reported | 1.0 | Heat exposure | (Laburn et al., 1988) |
| Sheep Ovis aries (4) | Not reported | 1.0 | Febrile, induced | (Laburn et al., 1988) |
| Sheep Ovis aries (4) | Not reported | 0.5 | Exercise | (Laburn et al., 1988) |
| Sheep Ovis aries (4) | Not reported | 0.8 | Heat exposure | (Nijland et al., 1990) |
| Sheep Ovis aries (4) | Not reported | 0.6 | Cold exposure | (Nijland et al., 1990) |
| Sheep Ovis aries (4) | Not reported | 0.9 | Febrile, induced | (Nijland et al., 1990) |
| Sheep Ovis aries (9) | 39.1 | 0.4 | Water deprivation and heat exposure | (Fuller et al., 2007) |
| Sheep Ovis aries (9) | 39.5 ± 0.5 | 1.5 | Water deprivation and heat exposure | (Strauss et al., 2016) |
| Sheep Ovis aries (5) | 39.1 ± 0.5 | 0.5 | (Maloney et al., 2007) | |
| Pig Sus scrofa (4) | 38.9 | 0.9 | Thermoneutral | (Fuller et al., 1999a) |
| Pig Sus scrofa (2) | Not reported | 0.8 | Heat stress | (Fuller et al., 1999a) |
| Pig Sus scrofa (1) | Not reported | 0.3 | Cold stress | (Fuller et al., 1999a) |
| Camel Camelus dromedarius (2) | 38.0 | 1.0 | At rest | (Schroter et al., 1989) |
| Camel Camelus dromedarius (1) | 39.5 | 1.5 | Exercise, hydrated and dehydrated | (Schroter et al., 1989) |
| Reindeer Rangifer tarandus (3) | 38.7 ± 0.2 | 1.0 | Heat exchanger | (Kuhnen and Mercer, 1993) |
| Reindeer Rangifer tarandus (3) | 39.5 ± 0.3 | 0.5 | Exercise | (Kuhnen and Mercer, 1993) |
| Thomson's gazelle Gazella thomsonii (5) | 39.4 | 2.7 | Exercise | (Taylor and Lyman, 1972) |
| Free-living wild animals with free access to normal behaviour | ||||
| Black wildebeest Connochaetes gnu (4) | 38.9 ± 0.2 | 0.4 | (Jessen et al., 1994) | |
| Eland Tragelaphus oryx (1) | 40.0 | 0.4 | (Fuller et al., 1999b) | |
| Gemsbok Oryx gazella (4) | 39.8 ± 0.4 | 0.4 | (Maloney et al., 2002) | |
| Gemsbok Oryx gazella (4) | 39.5 ± 0.9 | 0.9 | (Strauss et al., 2016) | |
| Kudu Tragelaphus strepsiceros (4) | 39.3 ± 0.7 | 0.5 | Febrile, naturally | (Hetem et al., 2008) |
| Kudu Tragelaphus strepsiceros (4) | 38.8 ± 0.1 | 0.2 | Afebrile | (Hetem et al., 2008) |
| Arabian oryx Oryx leucoryx (4) | 37.8 ± 0.1 | 1.4 | (Hetem et al., 2012) | |
| Springbok Antidorcas marsupialis (2) | 39.2 ± 0.2 | 0.5 | (Mitchell et al., 1997) | |
| Pronghorn Antilocapra americana (2) | 39.5 | 0.5 | (Lust et al., 2007) | |
| Blue wildebeest Connochaetes taurinus (6) | 39.3 ± 0.4 | 1.1 | (Strauss et al., 2016) | |
| Red hartebeest Alcelaphus buselaphus (5) | 39.4 ± 0.6 | 1.0 | (Strauss et al., 2016) | |
Non-thermal inputs
Cranial sympathetic tone
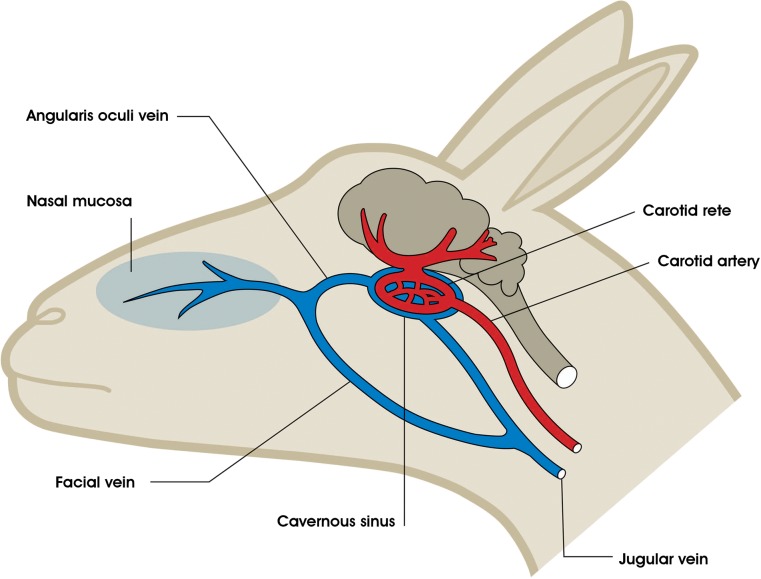
A role for sympathetic nervous system activity in the modulation of selective brain cooling was revealed through a series of elegant experiments on reindeer (Rangifer tarandus; Johnsen et al., 1985, 1987; Johnsen and Folkow, 1988). Experiments on habituated artiodactyls showed that selective brain cooling could be controlled by directing the passage of venous blood draining the evaporating surfaces of the head either to the cavernous sinus, classically via the angularis oculi veins, or to the jugular vein via the facial vein, bypassing the cavernous sinus (Fig. 3). The default direction of blood flow appears to be via the cavernous sinus, so increased cranial blood flow in heat-stressed artiodactyls (Maloney and Mitchell, 1997; Vesterdorf et al., 2011) led to increased flow of cooled venous blood to the cavernous sinus; hence, increased selective brain cooling. Increased cranial sympathetic nervous system activity, however, led to simultaneous contraction of a muscular sphincter in the angularis oculi vein (α-adrenergic) and dilatation of a similar sphincter in the facial vein (β-adrenergic), resulting in venous blood bypassing the cavernous sinus and returning via the jugular vein to the heart (Johnsen et al., 1985, 1987; Johnsen and Folkow, 1988). Although this differential vasoconstriction can modulate the degree of selective brain cooling, it does not fully explain the control of selective brain cooling in artiodactyls. In addition to the angularis oculi veins, less superficial veins, such as the sphenopalatine, external ophthalmic and ethmoidal veins, also supply venous blood to the cavernous sinus (Sisson and Grossman, 1967; Carlton and McKean, 1977). As a result, severing the angularis oculi veins does not completely eliminate selective brain cooling (Fuller et al., 2011). High sympathetic tone attenuates selective brain cooling not only by constricting the angularis oculi veins, but also by constricting nasal mucosal blood vessels and closing arteriovenous anastomoses. Within the nasal mucosa, the rate of heat extraction is attenuated through a combination of reduced blood flow and the restriction of airway width (Malm, 1973). Thus, increased cranial sympathetic nervous system activity decreases blood flow to the evaporating surfaces of the head as well as redirecting the flow of venous blood leaving those surfaces away from the cavernous sinus (Maloney and Mitchell, 1997; Fuller et al., 2011). These changes result in an upward shift of the threshold temperature for selective brain cooling, as documented in springbok (Antidorcas marsupialis; Mitchell et al., 1997), or complete abolishment of selective brain cooling, as documented in black wildebeest (Connochaetes gnu; Jessen et al., 1994). Free-living mammals rarely engage in intensive exercise except for predator–prey interactions, during which there is a dramatic increase in sympathetic nervous system activity in both predator and prey. The sympathetic activity prevalent during flight and fright in wild artiodactyls abolishes selective brain cooling (Jessen et al., 1994), overriding the drive of high body temperature. Selective brain cooling observed in tame artiodactyls exercising at low or moderate intensity is likely to be associated with low sympathetic activity (Mitchell et al., 2002).
Figure 3:
Diagram illustrating the position of the carotid rete in artiodactyls, located within a cavernous sinus at the base of the brain, as well as the main arterial blood supply to the brain, via the carotid rete. During selective brain cooling, cool venous blood from the nasal mucosa drains into the cavernous sinus via the angularis oculi vein (as well as some deeper veins; see Fuller et al., 2011). During high sympathetic activity, the cooled venous blood draining from the nasal mucosa largely bypasses the cavernous sinus as it is shunted via the facial vein, thereby attenuating selective brain cooling. Diagram adapted from Jessen (1998).
Although it is not as strong as in intense exercise during predator–prey interactions, there is also sympathetic activation during psychological stress. The effects of psychological stress, including an increase in body and brain temperatures, are well documented, even in mammals lacking a carotid rete, such as rats (Mohammed et al., 2014). In mammals with a carotid rete, selective brain cooling is absent or reduced in other circumstances likely to be associated with increased sympathetic tone, including nearby human presence (Maloney et al., 2001), the return of drinking water to dehydrated artiodactyls (Fuller et al., 2007) and vigilance in male artiodactyls (Maloney et al., 2002; Hetem et al., 2012). The temperament of individual mammals, a heritable trait among artiodactyls (Murphy et al., 1994), plays an important role in how situations are experienced (Beausoleil et al., 2008). For example, individual roe deer (Capreolus capreolus) manifest different responses to stress (Monestier et al., 2016) and therefore, presumably, different levels of sympathetic activation. Individual variability in sympathetic responses to the same stressor may contribute to an underlying plasticity in the control of selective brain cooling (Strauss et al., 2016).
Hydration status
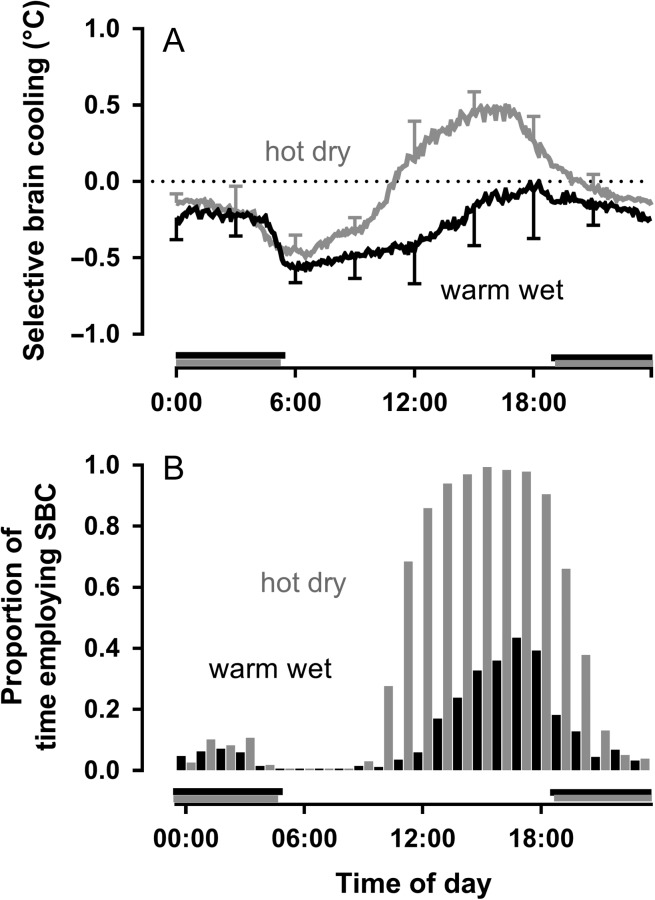
Selective brain cooling is enhanced during water deprivation (Jessen et al., 1998; Fuller et al., 2007; Strauss et al., 2015). Given that body temperature is elevated in dehydrated mammals exposed to heat, that enhancement could arise from a stronger thermal drive on selective brain cooling (Jessen et al., 1998). However, Fuller et al. (2007) showed that sheep exhibited a higher magnitude of selective brain cooling during dehydration even when carotid arterial blood temperature did not increase. Water deprivation on its own therefore seems to be a sufficient stimulus to enhance selective brain cooling. That idea is supported by measurement of selective brain cooling in antelope living free in arid environments. In the hyper-arid desert of Saudi Arabia, the mean magnitude of selective brain cooling in free-living Arabian oryx (Oryx leucoryx) peaked in the afternoon (Fig. 4A), well after solar noon and maximal heat load, as measured with miniature black globe thermometers on the collar of the animals (Hetem et al., 2007). Water availability, or aridity, appeared to have been the main factor determining the use and magnitude of selective brain cooling, with selective brain cooling being enhanced in the dry period compared with the wet period (Hetem et al., 2012). During the hot dry period when no drinking water was available, the Arabian oryx made near-continuous use of selective brain cooling during the 4 h leading to sunset (Fig. 5B, grey bars), compared with no more than 40% of the time during the warm wet period, when the oryx presumably had access to drinking water or food with a higher moisture content (Fig. 4B, black bars). Despite being exposed to similar ambient temperatures, Arabian oryx, in the hyper-arid desert of Saudi Arabia, also showed enhanced selective brain cooling compared with the congeneric gemsbok with free access to water (Maloney et al., 2002), as they initiated selective brain cooling at a lower threshold temperature and used selective brain cooling more frequently and at greater magnitude than did the gemsbok (Hetem et al., 2012).
Figure 4:
The effect of aridity on selective brain cooling as illustrated through differences in the mean (±SD) magnitude of selective brain cooling (carotid blood temperature minus hypothalamic temperature; A) and the proportion of time that a single Arabian oryx used selective brain cooling in the hot, hyper-arid deserts of Saudi Arabia (B). Grey depicts the hot dry and black the warm wet periods. Horizontal grey and black bars depict night-time during the two periods of interest. Data from Hetem et al. (2012).
Figure 5:
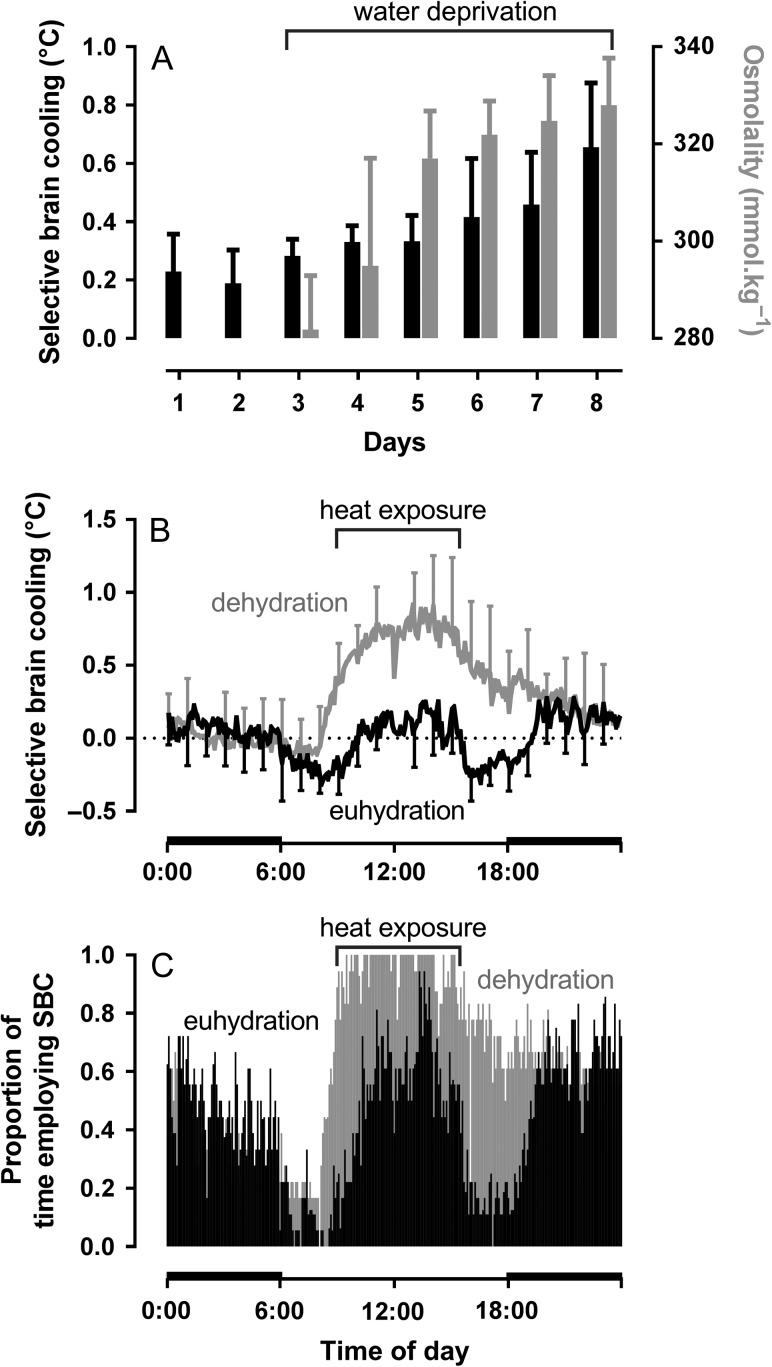
Summary of the effects of water deprivation on selective brain cooling in Dorper sheep. (A) The relationship between the mean (±SD) magnitude of selective brain cooling (carotid blood temperature minus hypothalamic temperature, black bars) and osmolality (grey bars) during 5 days of water deprivation; the black bracket indicates the period of water deprivation. (B) The mean (±SD) 24 h magnitude of selective brain cooling during euhydration (black line, days 1 and 2 in A) and dehydration (grey line, days 7 and 8 in A); horizontal black bars along the x-axis depict night-time. (C) The proportion of time that the sheep spent using selective brain cooling throughout the 24 h period when euhydrated (black bars; days 1 and 2 in A) and when dehydrated (grey bars; days 7 and 8 in A); the black bracket indicates the period of heat exposure (09:00–15:00) and the horizontal black bars along the x-axis depict night-time. Data from Strauss et al. (2015).
The enhancement of selective brain cooling by water deprivation has been confirmed in more controlled conditions, in which domestic artiodactyls can be handled for sample collection (Jessen et al., 1998; Fuller et al., 2007; Strauss et al., 2015). When Dorper sheep (Ovis aries) were deprived of drinking water and exposed to diurnal heat load, after the third day of water deprivation, when the water reservoir within the rumen would have been depleted, plasma osmolality and selective brain cooling magnitude increased in a near-linear manner and in unison (Fig. 5A; Strauss et al., 2015). After 5 days of water deprivation, the 24 h mean magnitude of selective brain cooling was three times greater (0.9 vs. 0.3°C) than that during euhydration (Fig. 5B). The proportion of time during which the sheep used selective brain cooling also differed markedly depending on hydration status. Figure 5C shows that the sheep used some selective brain cooling at all times of the day regardless of hydration status. However, when dehydrated, the sheep used selective brain cooling more frequently at all times of day, but especially during the daylight hours when selective brain cooling, on average, was used 75% of the time. Water deprivation, presumably acting through hyperosmolality, therefore appears to be a driver of selective brain cooling.
Selective brain cooling conserves body water
In dehydrated mammals, increased osmotic pressure of the arterial blood perfusing the brain inhibits thermoregulatory responses to heat (McKinley et al., 2008), including evaporative heat loss (Doris, 1983). If hyperosmolality also enhances selective brain cooling, then what would be the benefit to artiodactyls? In goats, at least, but presumably also in other artiodactyls, the neural drive for respiratory evaporative heat loss is provided about equally by thermoreceptors in the hypothalamus and thermoreceptors within the trunk (Jessen and Feistkorn, 1984). During selective brain cooling, input from the hypothalamic temperature sensors would be attenuated. Consequently, panting and sweating, the main physiological avenues of evaporative heat loss in artiodactyls, would be attenuated. The potential water savings as a result of selective brain cooling could be substantial considering that a 1°C decrease in brain temperature resulted in a ~6-fold decrease in respiratory evaporative water loss in goats (Kuhnen and Jessen, 1994). The hypothalamic thermosensitivity for evaporative water loss in goats is therefore high. We calculated the hypothalamic thermosensitivity for evaporative water loss for goats, at a trunk temperature of 40°C, to be 0.35 W kg−1 °C−1, or 14 g H2O min−1 kg−1 °C−1 using data provided in Jessen and Feistkorn (1984). In contrast, for a species lacking a rete, the rabbit, the hypothalamic thermosensitivity for evaporative water loss, calculated at an ambient temperature of 39°C, was 0.038 W kg−1 °C−1, or 1.5 g H2O min−1 kg−1 °C−1 (Stitt, 1976). The hypothalamic thermosensitivity is therefore 10 times greater in the heat-exposed goat than in the heat-exposed rabbit. A relatively small change in the hypothalamic temperature of the goat (tenths of a degree Celsius) therefore can result in significant water savings as a result of decreased evaporative water loss.
In an elegant experiment on goats, Kuhnen (1997) used extracorporeal heat exchangers to manipulate selective brain cooling and measured respiratory evaporative heat loss. The experimental inhibition of selective brain cooling resulted in a reduced trunk threshold for respiratory evaporative heat loss; therefore, evaporative water loss occurred at lower body temperatures, as well as at a higher overall rate. At an aortic blood temperature of 40°C, selective brain cooling of only 0.5°C reduced respiratory water loss by 0.72 l day−1, the equivalent of 35% of the average daily water requirement of the goats (Kuhnen, 1997). Sweating also is driven largely by thermal receptors in the hypothalamus (Smiles et al., 1976); therefore, selective brain cooling also will reduce water loss by sweating (Strauss et al., 2015), further contributing to the water savings of a mammal that uses both forms of evaporative heat loss.
Recently, in the laboratory, we quantified the total water savings attributable to selective brain cooling in Dorper sheep. We used the stable hydrogen isotope deuterium oxide (D2O) to measure water turnover in sheep that were deprived of drinking water and naturally making use of selective brain cooling. The sheep lost a quarter of their body water over 5 days of water deprivation. The threshold temperature for selective brain cooling remained unchanged, but those individuals that used selective brain cooling more frequently, or of greater magnitude, had lower water turnover rates (they therefore conserved body water better) than did conspecifics that used selective brain cooling less frequently or of smaller magnitude. We showed that a 50 kg sheep that used selective brain cooling for half of a day would save 2.4 litres of water that day, the equivalent of ~60% of the daily water requirement of a Dorper sheep not exposed to heat (Strauss et al., 2015).
Thus, the reduction of hypothalamic temperature by the 1°C or less that selective brain cooling can achieve reduces evaporative cooling during heat exposure sufficiently to save a substantial portion of the water that an artiodactyl would need to access each day. How does an artiodactyl continue to maintain heat balance if its evaporative cooling is attenuated? The attenuation of evaporative cooling by selective brain cooling results in an increase in body temperature, including skin temperature (Caputa et al., 1986b; Laburn et al., 1988). In environments in which an artiodactyl can lose heat by radiation and convection, the higher skin temperature will enhance radiant and convective heat loss, so selective brain cooling will switch heat loss from evaporative to non-evaporative channels. If an artiodactyl is in an environment in which it is gaining heat, the rate of heat gain will be reduced by the higher skin temperature. In the process of conserving water, however, the artiodactyl may store heat during hot periods of the day. As selective brain cooling generally is used late in the afternoon (Fig. 2, left panels), the heat that is gained as a result of the suppression of evaporative cooling may be dissipated non-evaporatively during the night.
Selective brain cooling as a physiological feature for surviving hotter and drier environments
Water economy strategies in artiodactyls provide compelling evidence that selective brain cooling can mitigate negative population responses to warming and aridification. The use of selective brain cooling offers significant water savings to artiodactyls, but it is not the only mechanism by which they can save water. Dehydration itself reduces evaporative water loss via osmosensitive neurons in the hypothalamus, even in the absence of selective brain cooling (Baker and Doris, 1982). However, unlike dehydration or blood osmolality, selective brain cooling can be switched off rapidly (probably within seconds) by high cranial sympathetic activity. That property of selective brain cooling potentially conveys a survival benefit (Mitchell et al., 2002). For example, should an artiodactyl in a hot environment and implementing selective brain cooling be confronted by a flight-or-fight situation, its selective brain cooling would be abolished immediately by increased sympathetic tone. Consequently, hypothalamic temperature would increase, and the hypothalamic drive on evaporative cooling would be restored immediately, with the full power of evaporative cooling invoked to dissipate the extra metabolic heat. Immediate survival outweighs the longer-term benefits of body water conservation, and the artiodactyl makes temporary use of full evaporative cooling to avoid a potentially lethal hyperthermia. When the threat has receded, selective brain cooling can again be initiated and evaporative water loss suppressed. Thus, selective brain cooling may bestow benefits for survival in arid environments in two ways: (i) switching selective brain cooling on conserves body water, improving long-term survival; whereas (ii) switching selective brain cooling off rapidly accelerates evaporative cooling to avoid lethal hyperthermia, thereby supporting immediate survival.
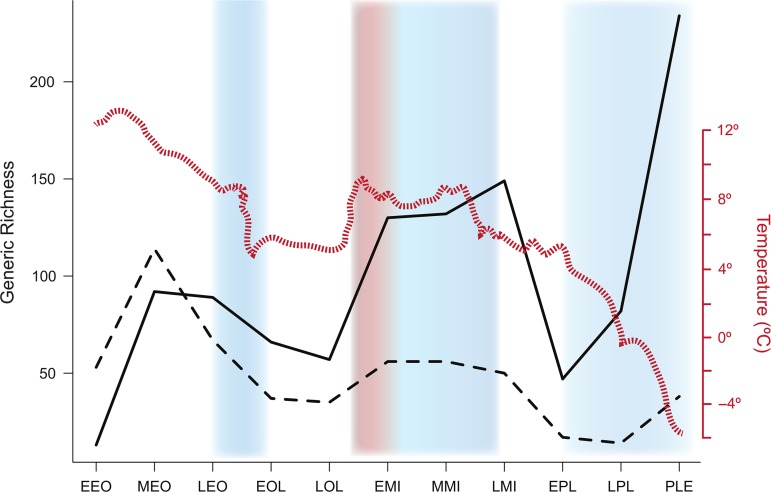
The ability to modulate evaporative water loss through the use of selective brain cooling may have contributed to the evolutionary success of artiodactyls (Mitchell and Lust, 2008). It can be assumed that the carotid rete, and therefore selective brain cooling, evolved concomitantly with the emergence of the modern artiodactyl orders, at least 45 million years ago (Janis, 2009). When the diversity trends of Artiodactyla (presumably all rete bearing) and their sister clade Perissodactyla (presumably not rete bearing) are compared across the Cenozoic, discrepancies in generic richness are established in three pulses, each of which corresponds to a trend of aridification, whether warming or cooling (Fig. 6). In the earlier half of the Cenozoic, global climate is best described as a ‘tropical hothouse’ (Buchardt, 1978; Wolfe, 1978; Wing, 1987; Huber and Sloan, 2001; Bowen and Zachos, 2010; Galeotti et al., 2010). During this period of abundant moisture, artiodactyls and perissodactyls enjoyed similar generic richness (Fig. 6). However, a pronounced period of cooling and drying across the Eocene to Oligocene transition began to shift this relationship (Diester-Haass and Zahn, 1996; Lear et al., 2008). Although many large-bodied mammals faced extinction throughout this period (Prothero, 1985; Hooker, 1992; Legendre and Hartenberger, 1992), artiodactyls enjoyed a comparatively higher degree of survivorship both during and after this event. Throughout the drier, temperate Oligocene (Kennett, 1985; Ehrmann and Mackensen, 1992), artiodactyl generic richness remained elevated relative to perissodactyls (Fig. 6). Across the Late Oligocene to Early Miocene transition, another period of aridification swept the globe, this time accompanied by warming (Kennett, 1985; Retallack, 2013). This period of warming and aridification saw a pulse in generic richness of artiodactyls capable of selective brain cooling, immediately before the Mid-Miocene expansive radiation of C4 grasslands. It is often the expansion of grasslands and the artiodactyl rumen that have been associated and promoted as the driver of artiodactyl success (Retallack, 1991, 2001, 2007a, b, 2013; Jacobs et al., 1999). Although the late Cenozoic (Mid-Miocene to present) diversification patterns are largely attributable to the ruminant digestive physiology of bovids (Janis et al., 1998; Clauss et al., 2003; Janis, 2007, 2009; Clauss and Rössner, 2014; O'Brien, 2016), this highly specialized mode of digestion is variably present and cannot solely be responsible for earlier Cenozoic survivorship and diversification patterns. Indeed, it is likely that the influence of selective brain cooling insulated artiodactyls from extensive periods of aridification, whether warming or cooling, that saw declines among non-selective-brain-cooling ungulates (O'Brien, 2016).
Figure 6:
Diversity curve for the artiodactyls (solid line) and perissodactyls (dashed line) from the early Eocene (EEO) to the Palaeocene (PLE) relative to global temperature (red line; Zachos et al., 2001) and an indication of the relative hydrological regimes (Janis, 2008); blue and red shading represent aridification combined with cooling and warming temperatures, respectively. Occurrence data were downloaded from the Fossilworks/Paleobiology Database in August 2016. The epochs along the x-axis are as follows: EEO, Early Eocene; MEO, Mid-Eocene; LEO, Late Eocene; EOL, Early Oligocene; LOL, Late Oligocene; EMI, Early Miocene; MMI, Mid-Miocene; LMI, Late Miocene; EPL, Early Pleistocene; LPL, Late Pleistocene; and PLE, Paleocene.
This pattern of selective-brain-cooling-related survivorship is corroborated by evolutionary patterns of the Tragulidae. Although they are small-bodied artiodactyls with a rudimentary capacity for rumination, tragulids do not possess a carotid rete. Therefore, they can be considered to ruminate but not to selective brain cool. The earliest record of tragulids and other closely related small-bodied primitive ruminants is the Late Eocene to Early Oligocene (Webb and Taylor, 1980), when swamps and temperate forests dominated much of Europe and North America (Mai, 1989; Pross et al., 2001; Retallack, 2007a, b, 2013; Kunzmann, 2012). Since that period, these non-selective-brain-cooling taxa have experienced declines in generic richness even as more advanced ruminants have increased in diversity (Clauss and Rössner, 2014). Thus, although diet undoubtedly plays a role in the extinction and survivorship capacity of any taxon, failure to incorporate selective brain cooling not only leaves early artiodactyl evolution unsatisfactorily explained, but it renders an incomplete evaluation of these species’ abilities to persist into the Anthropocene.
Among artiodactyl species, there may be innate differences between species in their capacity for selective brain cooling, which may facilitate the persistence of those species with enhanced selective brain cooling in a hotter and drier Anthropocene. Although it has not been possible logistically, to date, to measure the water savings that result from selective brain cooling in free-living artiodactyls, we recently investigated selective brain cooling in three sympatric antelope species with different water dependencies and determined the dimensions of their carotid retes (Strauss et al., 2016). Individuals of all three species, living free in their natural habitats under the same environmental conditions, used selective brain cooling, but we found no differences in selective brain cooling use between the gemsbok that is independent of surface water, the red hartebeest of intermediate water dependency and the blue wildebeest that is dependent on surface water and has to drink water daily. In fact, we found more variability in selective brain cooling use within those species than between those species in a habitat in which surface water was available ad libitum. Although earlier descriptions of carotid rete morphology documented variability in the vascularization of the rete between species (Daniel et al., 1953; Carlton and McKean, 1977), we found little quantifiable difference in the rete anatomy of those three artiodactyls with varying ecological water requirements (Strauss et al., 2016). The observed variability in use of selective brain cooling within these three species living in exactly the same environmental conditions reaffirms the concept that selective brain cooling is not simply under thermal control (in the sense that there is a threshold temperature at which it is initiated). Moreover, the plasticity in the use of selective brain cooling may provide a physiological feature for selection in the face of anthropogenic climate change, as numerous regions are expected to become hotter and drier, with increased variability in rainfall (Niang et al., 2014). Large mammals will be unable to adapt genetically given the rapid rate of climate change and will be unlikely to be able to move to new, suitable habitats, leaving them dependent on phenotypic plasticity if they are to counter such climate change (Hetem et al., 2014).
Having survived and diversified dramatically during periods of pronounced aridification, the artiodactyls may be insulated, to a degree, from global warming and drying (Mitchell and Lust, 2008). Moreover, the observed inter-individual variability in selective brain cooling use within species implies that individual artiodactyls within populations, regardless of species or ecological water dependency, might have a relative evolutionary advantage in hotter, drier, less predictable environments. Representatives of the characteristic large herds of artiodactyls across various landscapes, which often also form the mainstay of large tracts of land under conservation management, therefore could persist through the Anthropocene. However, considering the continuing decrease in antelope populations across the globe (Ripple et al., 2015) and the poor performance of artiodactyls during mid-latitude glaciation of the Miocene to Pliocene transition, persistence through the Anthropocene may depend on the degree of intra-specific selective brain cooling plasticity. Indeed, artiodactyls such as the Arabian oryx may already be living close to their physiological tolerance levels. With a 24 h body temperature amplitude (maximum minus minimum 24 h body temperature) of up to 7.7°C during the summer, these animals are seasonally losing control of homeothermy as a result of water and nutritional stress (Hetem et al., 2012, 2016). As a consequence of a paucity of similar data from other artiodactyls, and also from perissodactyls, we know little about the physiological performance of these mammals under current environmental conditions and, ultimately, their ability to cope with a changing climate. Because perissodactyls, many of which are evolutionarily distinct (Zoological Society of London, 2014), cannot use selective brain cooling to conserve body water, concerted conservation efforts may be required as conditions become drier under anthropogenic climate change. Yet such conservation efforts should not be to the detriment of other species sensitive to disturbance (see, for example, Harrington et al., 1999).
Survival of those predators depredating artiodactyls and perissodactyls may also depend on the predators’ capacity for selective brain cooling, as selective brain cooling has been observed in heat-stressed domestic cats (Baker and Doris, 1982) and dogs (Baker, 1984). The degree to which free-living felids and canids use selective brain cooling is currently not known. We also do not know, therefore, whether the evolutionary success of carnivores can be attributed to the development of their carotid rete, selective brain cooling and the conservation of body water. A better understanding of selective brain cooling, as a form of physiological plasticity that is available to some mammals, is integral to efforts to predict how mammals will respond to changing environments and how best to conserve them. The studies that are required are long-term investigations undertaken on a range of artiodactyl and carnivore species (Fuller et al., 2014; Hetem et al., 2014). Such studies should focus on identifiable individuals and their progeny, free-living in their natural environment, with the aim of relating varying levels of evolutionary success to the observed flexibility in selective brain cooling and resulting water savings.
Acknowledgements
We thank the managers and owners of numerous field sites for allowing us to access their facilities, and providing invaluable support during our field studies. The staff at the Central Animal Services, University of the Witwatersrand, are thanked for their assistance and support. We thank Glenn Tattersall for commenting on an earlier version of this paper
Funding
We thank the South African National Research Foundation (NRF), the Carnegie Corporation of New York, the Global Change SysTem for Analysis, Research and Training (START), the Oppenheimer Memorial Trust, the University of the Witwatersrand, the University of South Africa and the British Ecological Society (BES) for financial support of our research described in this review.
References
- Ask-Upmark E. (1935) The carotid sinus and the cerebral circulation. Acta Psychiatr Neurol 6 (supplement). [Google Scholar]
- Baker MA. (1972) Influence of the carotid rete on brain temperature in cats exposed to hot environments. J Physiol 220: 711–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MA. (1979) A brain cooling system in mammals. Sci Am 240: 114–123. [DOI] [PubMed] [Google Scholar]
- Baker MA. (1984) Cardiovascular and respiratory responses to heat in dehydrated dogs. Am J Physiol Regul Integr Comp Physiol 246: R369–R374. [DOI] [PubMed] [Google Scholar]
- Baker MA, Chapman LW (1977) Rapid brain cooling in exercising dogs Science 195: 781–783. [DOI] [PubMed] [Google Scholar]
- Baker MA, Doris PA (1982) Effect of dehydration on hypothalamic control of evaporation in the cat. J Physiol 322: 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MA, Hayward JN (1967) Carotid rete and brain temperature of cats. Nature 216: 139–141. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hayward JN (1968) The influence of the nasal mucosa and the carotid rete upon hypothalamic temperature in sheep. J Physiol 198: 561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MA, Nijland MJ (1993) Selective brain cooling in goats: effects of exercise and dehydration. J Physiol 471: 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil NJ, Blache D, Stafford KJ, Mellor DJ, Noble ADL (2008) Exploring the basis of divergent selection for ‘temperament’ in domestic sheep. Appl Anim Behav Sci 109: 261–274. [Google Scholar]
- Bowen GJ, Zachos JC (2010) Rapid carbon sequestration at the termination of the Palaeocene–Eocene Thermal Maximum. Nat Geosci 3: 866–869. [Google Scholar]
- Braasch D. (1964) Zur Pathogenese des tödlichen Kreislaufkollapses nach Überwärmung einzelner Organe auf 45°C. Pflügers Archiv 278: 567–574. [PubMed] [Google Scholar]
- Buchardt B. (1978) Oxygen isotope palaeotemperatures from the Tertiary period in the North Sea area. Nature 275: 121–123. [Google Scholar]
- Burger FJ, Fuhrman FA (1964) Evidence of injury by heat in mammalian tissues. Am J Physiol 206: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Caputa M, Feistkorn G, Jessen C (1986. a) Competition for cool nasal blood between trunk and brain in hyperthermic goats. Comp Biochem Physiol A Comp Physiol 85: 423–427. [DOI] [PubMed] [Google Scholar]
- Caputa M, Feistkorn G, Jessen C (1986. b) Effects of brain and trunk temperatures on exercise performance in goats. Pflügers Archiv 406: 184–189. [DOI] [PubMed] [Google Scholar]
- Carlton C, McKean T (1977) The carotid and orbital retia of the pronghorn, deer and elk. Anat Rec 189: 91–107. [DOI] [PubMed] [Google Scholar]
- Chapman HC. (1881) Observations upon the hippopotamus. Proc Acad Natl Sci Phila 33: 126–148. [Google Scholar]
- Chesy G, Caputa M, Kędziela W, Kozak W, Lachowski A (1983) The influence of ambient temperature on brain homeothermia in the ox (Bos taurus). J Therm Biol 8: 259–263. [Google Scholar]
- Chesy G, Caputa M, Kędziela W, Kozak W, Lachowski A (1985) Selective brain cooling in the ox (Bos taurus) during heavy exercise. J Therm Biol 10: 57–61. [Google Scholar]
- Clauss M, Rössner GE (2014) Old world ruminant morphophysiology, life history, and fossil record: exploring key innovations of a diversification sequence. Ann Zool Fenn 51: 80–94. [Google Scholar]
- Clauss M, Frey R, Kiefer B, Lechner-Doll M, Loehlein W, Polster C, Rössner GE, Streich WJ (2003) The maximum attainable body size of herbivorous mammals: morphophysiological constraints on foregut, and adaptations of hindgut fermenters. Oecologia 136: 14–27. [DOI] [PubMed] [Google Scholar]
- Daniel PM, Dawes JDK, Prichard MML (1953) Studies of the carotid rete and its associated arteries. Philos Trans R Soc Lond B Biol Sci 237: 173–208. [Google Scholar]
- Diester-Haass L, Zahn R (1996) Eocene-Oligocene transition in the Southern Ocean: History of water mass circulation and biological productivity. Geology 24: 163–166. [Google Scholar]
- Doris PA. (1983) Osmotic regulation of evaporative water loss and body temperature by intracranial receptors in the heat-stressed cat. Pflugers Archiv 398: 337–340. [DOI] [PubMed] [Google Scholar]
- du Boulay GH, Verity PM (1973) The Cranial Arteries of Mammals Williman Heinemann Medical Books Ltd, London. [Google Scholar]
- Ehrmann WU, Mackensen A (1992) Sedimentological evidence for the formation of an East Antarctic ice sheet in Eocene/Oligocene time. Palaeogeogr Palaeoclimatol Palaeoecol 93: 85–112. [Google Scholar]
- Frąckowiak H. (2006) The artery of the head in some mammalian orders In Zgrabczyńska ĆP, Ziomek J, eds, Animals, Zoos and Conservation: Zoological Garden in Poznan. Kontekst Publisher House, Poznań, pp 171–180. [Google Scholar]
- Frąckowiak H, Debinski D, Komosa M, Zdun M (2015) The arterial circle of the brain, its branches and connections in selected representatives of the Antilopinae. J Morphol 276: 766–771. [DOI] [PubMed] [Google Scholar]
- Fukuta K, Kudo H, Sasaki M, Kimura J, bin Ismail D, Endo H (2007) Absence of carotid rete mirabile in small tropical ruminants: implications for the evolution of the arterial system in artiodactyls. J Anat 210: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A, Mitchell G, Mitchell D (1999. a) Non-thermal signals govern selective brain cooling in pigs. J Comp Physiol B 169: 605–611. [DOI] [PubMed] [Google Scholar]
- Fuller A, Moss DG, Skinner JD, Jessen PT, Mitchell G, Mitchell D (1999. b) Brain, abdominal and arterial blood temperatures of free-ranging eland in their natural habitat. Pflugers Archiv 438: 671–680. [DOI] [PubMed] [Google Scholar]
- Fuller A, Maloney SK, Kamerman PR, Mitchell G, Mitchell D (2000) Absence of selective brain cooling in free-ranging zebras in their natural habitat. Exp Physiol 85: 209–217. [PubMed] [Google Scholar]
- Fuller A, Meyer LCR, Mitchell D, Maloney SK (2007) Dehydration increases the magnitude of selective brain cooling independently of core temperature in sheep. Am J Physiol Regul Integr Comp Physiol 293: R438–R446. [DOI] [PubMed] [Google Scholar]
- Fuller A, Hetem RS, Meyer LCR, Maloney SK (2011) Angularis oculi vein blood flow modulates the magnitude but not the control of selective brain cooling in sheep. Am J Physiol Regul Integr Comp Physiol 300: R1409–R1417. [DOI] [PubMed] [Google Scholar]
- Fuller A, Hetem RS, Maloney SK, Mitchell D (2014) Adaptation to heat and water shortage in large, arid-zone mammals. Physiology 29: 159–167. [DOI] [PubMed] [Google Scholar]
- Galeotti S, Krishnan S, Pagani M, Lanci L, Gaudio A, Zachos JC, Monechi S, Morelli G, Lourens L (2010) Orbital chronology of Early Eocene hyperthermals from the Contessa Road section, central Italy. Earth Planet Sci Lett 290: 192–200. [Google Scholar]
- Gillilan LA. (1974) Blood supply to brains of ungulates with and without a rete mirabile caroticum. J Comp Neurol 153: 275–290. [DOI] [PubMed] [Google Scholar]
- Gillilan LA. (1976) Extra- and intra-cranial blood supply to brains of dog and cat. Am J Anat 146: 237–253. [DOI] [PubMed] [Google Scholar]
- Harrington R, Owen-Smith N, Viljoen PC, Biggs HC, Mason DR, Funston P (1999) Establishing the causes of the roan antelope decline in the Kruger National Park, South Africa. Biol Conserv 90: 69–78. [Google Scholar]
- Hassanin A, Delsuc F, Ropiquet A, Hammer C, Jansen van Vuuren B, Matthee C, Ruiz-Garcia M, Catzeflis F, Areskoug V, Nguyen TT et al. (2012) Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. C R Biol 335: 32–50. [DOI] [PubMed] [Google Scholar]
- Hayward JN, Smith E, Stuart DG (1966) Temperature gradients between arterial blood and brain in the monkey. Proc Soc Exp Biol Med 121: 547–551. [DOI] [PubMed] [Google Scholar]
- Hebert J, Lust A, Fuller A, Maloney SK, Mitchell D, Mitchell G (2008) Thermoregulation in pronghorn antelope (Antilocapra americana, Ord) in winter. J Exp Biol 211: 749–756. [DOI] [PubMed] [Google Scholar]
- Hetem RS, Maloney SK, Fuller A, Meyer LCR, Mitchell D (2007) Validation of a biotelemetric technique, using ambulatory miniature black globe thermometers, to quantify thermoregulatory behaviour in ungulates. J Exp Zool A Ecol Genet Physiol 307A: 342–356. [DOI] [PubMed] [Google Scholar]
- Hetem RS, Mitchell D, Maloney SK, Meyer LCR, Fick LG, Kerley GIH, Fuller A (2008) Fever and sickness behavior during an opportunistic infection in a free-living antelope, the greater kudu (Tragelaphus strepsiceros). Am J Physiol Regul Integr Comp Physiol 294: R246–R254. [DOI] [PubMed] [Google Scholar]
- Hetem RS, Strauss WM, Fick LG, Maloney SK, Meyer LCR, Fuller A, Shobrak M, Mitchell D (2012) Selective brain cooling in Arabian oryx (Oryx leucoryx): a physiological mechanism for coping with aridity. J Exp Biol 215: 3917–3924. [DOI] [PubMed] [Google Scholar]
- Hetem RS, Fuller A, Maloney SK, Mitchell D (2014) Responses of large mammals to climate change. Temperature 1: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetem RS, Maloney SK, Fuller A, Mitchell D (2016) Heterothermy in large mammals: inevitable or implemented. Biol Rev 91: 187–205. [DOI] [PubMed] [Google Scholar]
- Hooker JJ. (1992) British mammalian paleocommunities across the Eocene-Oligocene transition and their environmental implications In Prothero DR, Berggren WA, eds, Eocene-Oligocene Climatic and Biotic Evolution. Princeton University Press, Princeton, pp 494–511. [Google Scholar]
- Huber M, Sloan LC (2001) Heat transport, deep waters, and thermal gradients: coupled simulation of an Eocene greenhouse climate. Geophys Res Lett 28: 3481–3484. [Google Scholar]
- Jacobs BF, Kingston JD, Jacobs LL (1999) The origin of grass-dominated ecosystems. Ann Missouri Bot Gard 86: 590–643. [Google Scholar]
- Janis CM. (2008) An evolutionary history of grazing and browsing In Gordon IJ, Prins HTT, eds, The Ecology of Browsing and Grazing, Vol 195 of the series Ecological Studies. Springer, Berlin Heidelberg, pp 21–45. [Google Scholar]
- Janis C. (2009) Artiodactyl ‘success’ over perissodactyls in the late Palaeogene unlikely to be related to the carotid rete: a commentary on Mitchell & Lust (2008). Biol Lett 5: 97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis CM. (1984) Tragulids as living fossils In Eldredge N, Stanley S, eds, Living Fossils. Springer, New York, pp 87–94. [Google Scholar]
- Janis CM. (2007) Evolutionary patterns and paleobiology In Prothero DR, Foss SE, eds, The Evolution of Artiodactyls. Johns Hopkins University Press, Baltimore, pp 292–302. [Google Scholar]
- Janis CM, Scott KM, Jacobs LL (1998) Evolution of Tertiary Mammals of North America, Vol 1, Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals. Cambridge University Press, Cambridge. [Google Scholar]
- Jessen C. (1998) Brain cooling: an economy mode of temperature regulation in artiodactyls. News Physiol Sci 13: 281–286. [DOI] [PubMed] [Google Scholar]
- Jessen C, Feistkorn G (1984) Some characteristics of core temperature signals in the conscious goat. Am J Physiol Regul Integr Comp Physiol 247: R456–R464. [DOI] [PubMed] [Google Scholar]
- Jessen C, Laburn HP, Knight MH, Kuhnen G, Goelst K, Mitchell D (1994) Blood and brain temperatures of free-ranging black wildebeest in their natural environment. Am J Physiol Regul Integr Comp Physiol 267: R1528–R1536. [DOI] [PubMed] [Google Scholar]
- Jessen C, Dmi'el R, Choshniak I, Ezra D, Kuhnen G (1998) Effects of dehydration and rehydration on body temperatures in the black Bedouin goat. Pflugers Archiv 436: 659–666. [DOI] [PubMed] [Google Scholar]
- Johnsen HK, Folkow LP (1988) Vascular control of brain cooling in reindeer. Am J Physiol Regul Integr Comp Physiol 254: R730–R739. [DOI] [PubMed] [Google Scholar]
- Johnsen HK, Blix AS, Jørgensen L, Mercer JB (1985) Vascular basis for regulation of nasal heat exchange in reindeer. Am J Physiol Regul Integr Comp Physiol 249: R617–R623. [DOI] [PubMed] [Google Scholar]
- Johnsen HK, Blix AS, Mercer JB, Bolz KD (1987) Selective cooling of the brain in reindeer. Am J Physiol Regul Integr Comp Physiol 253: R848–R853. [DOI] [PubMed] [Google Scholar]
- Kamijyo Y, Garcia JH (1975) Carotid arterial supply of the feline brain. Stroke 6: 361–369. [DOI] [PubMed] [Google Scholar]
- Kennett JP. (1985) Neogene paleoceanography and plankton evolution. S Afr J Sci 81: 251–253. [Google Scholar]
- Kiełtyka-Kurc A, Frąckowiak H, Brudnicki W (2015) The arteries of brain base in species of the Cervid family. Anat Rec 298: 735–740. [DOI] [PubMed] [Google Scholar]
- Kuhnen G. (1997) Selective brain cooling reduces respiratory water loss during heat stress. Comp Biochem Physiol A Comp Physiol 118: 891–895. [DOI] [PubMed] [Google Scholar]
- Kuhnen G, Jessen C (1991) Threshold and slope of selective brain cooling. Pflugers Archiv 418: 176–183. [DOI] [PubMed] [Google Scholar]
- Kuhnen G, Jessen C (1992) Effects of selective brain cooling on mechanisms of respiratory heat loss. Pflügers Archiv 421: 204–208. [DOI] [PubMed] [Google Scholar]
- Kuhnen G, Jessen C (1994) Thermal signals in control of selective brain cooling. Am J Physiol Regul Integr Comp Physiol 267: R355–R359. [DOI] [PubMed] [Google Scholar]
- Kuhnen G, Mercer JB (1993) Selective brain cooling in resting and exercising Norwegian reindeer (Rangifer tarandus tarandus). Acta Physiol Scand 147: 281–288. [DOI] [PubMed] [Google Scholar]
- Kunzmann L. (2012) Early Oligocene riparian and swamp forests with a mass occurrence of Zingiberoideophyllum (extinct Zingiberales) from Saxony, Central Germany. Palaios 27: 765–778. [Google Scholar]
- Laburn HP, Mitchell D, Mitchell G, Saffy K (1988) Effects of tracheostomy breathing on brain and body temperatures in hyperthermic sheep. J Physiol 406: 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear CH, Bailey TR, Pearson PN, Coxall HK, Rosenthal Y (2008) Cooling and ice growth across the Eocene-Oligocene transition. Geology 36: 251–254. [Google Scholar]
- Legendre S, Hartenberger J-L (1992) Evolution of mammalian faunas in Europe during the Eocene and Oligocene In Prothero DR, Berggren WA, eds, Eocene-Oligocene Climatic and Biotic Evolution. Princeton University Press, Princeton, pp 516–528. [Google Scholar]
- Leon LR, Helwig BG (2010) Heat stroke: role of the systemic inflammatory response. J Appl Physiol 109: 1980–1988. [DOI] [PubMed] [Google Scholar]
- Lust A, Fuller A, Maloney SK, Mitchell D, Mitchell G (2007) Thermoregulation in pronghorn antelope (Antilocapra americana Ord) in the summer. J Exp Biol 210: 2444–2452. [DOI] [PubMed] [Google Scholar]
- Magilton JH, Swift CS (1968) Description of two physiological heat exchange systems for the control of brain temperature. In IEEE Conference Record: Fifth Annual Rocky Mountain Bioengineering Symposium, pp 24–27.
- Mai DH. (1989) Development and regional differentiation of the European vegetation during the Tertiary. Plant Syst Evol 162: 79–91. [Google Scholar]
- Malm L. (1973) Stimulation of sympathetic nerve fibres to the nose in cats. Acta Otolaryngol 75: 519–526. [DOI] [PubMed] [Google Scholar]
- Maloney SK, Mitchell G (1997) Selective brain cooling: role of angularis oculi vein and nasal thermoreception. Am J Physiol Regul Integr Comp Physiol 273: R1108–R1116. [DOI] [PubMed] [Google Scholar]
- Maloney SK, Fuller A, Mitchell G, Mitchell D (2001) Rectal temperature measurement results in artifactual evidence of selective brain cooling. Am J Physiol Regul Integr Comp Physiol 281: R108–R114. [DOI] [PubMed] [Google Scholar]
- Maloney SK, Fuller A, Mitchell G, Mitchell D (2002) Brain and arterial blood temperatures of free-ranging oryx (Oryx gazella). Pflugers Archiv 443: 437–445. [DOI] [PubMed] [Google Scholar]
- Maloney SK, Mitchell D, Blache D (2007) The contribution of carotid rete variability to brain temperature variability in sheep in a thermoneutral environment. Am J Physiol Regul Integr Comp Physiol 292: R1298–R1305. [DOI] [PubMed] [Google Scholar]
- Maloney SK, Fuller A, Meyer LCR, Kamerman PR, Mitchell G, Mitchell D (2009) Brain thermal inertia, but no evidence for selective brain cooling, in free-ranging western grey kangaroos (Macropus fuliginosus). J Comp Physiol B 179: 241–251. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Whyte D, Mathai ML (2008) Central osmoregulatory influences on thermoregulation. Clin Exp Pharmacol Physiol 35: 701–705. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Laburn HP, Nijland MJ, Zurovsky Y (1987) Selective brain cooling and survival. S Afr J Sci 83: 598–604. [Google Scholar]
- Mitchell D, Maloney SK, Laburn HP, Knight MH, Kuhnen G, Jessen C (1997) Activity, blood temperature and brain temperature of free-ranging springbok. J Comp Physiol B 167: 335–343. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Maloney SK, Jessen C, Laburn HP, Kamerman PR, Mitchell G, Fuller A (2002) Adaptive heterothermy and selective brain cooling in arid-zone mammals. Comp Biochem Physiol B Biochem Mol Biol 131: 571–585. [DOI] [PubMed] [Google Scholar]
- Mitchell G, Lust A (2008) The carotid rete and artiodactyl success. Biol Lett 4: 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed M, Ootsuka Y, Blessing W (2014) Brown adipose tissue thermogenesis contributes to emotional hyperthermia in a resident rat suddenly confronted with an intruder rat. Am J Physiol Regul Integr Comp Physiol 306: R394–R400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monestier C, Gilot-Fromont E, Morellet N, Debeffe L, Cebe N, Merlet J, Picot D, Rames J-L, Hewison AJM, Verheyden H (2016) Individual variation in an acute stress response reflects divergent coping strategies in a large herbivore. Behav Process 132: 22–28. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Purvis IW, Lindsay DR, Le Neindre P, Orgeur P, Poindron P (1994) Measures of temperament are highly repeatable in Merino sheep and some are related to maternal behaviour. Proc Aust Soc Anim Prod 20: 247–254. [Google Scholar]
- Niang I, Ruppel OC, Abdrabo MA, Essel A, Lennard C, Padgham J, Urquhart P (2014) Africa In Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC et al., eds, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, NY, USA, pp 1199–1265. [Google Scholar]
- Nijland MM, Mitchell D, Mitchell G (1990) Selective brain cooling after bilateral superior cervical sympathectomy in sheep (Ovis aries). Pflugers Archiv 417: 375–381. [DOI] [PubMed] [Google Scholar]
- O'Brien HD. (2015) Cranial arterial pattern of the Sri Lankan spotted chevrotain, Moschiola memmina, and comparative basicranial osteology of the Tragulidae. Peer J 3: e1451 doi. org/1410.7717/peerj.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien HD. (2016) Macroevolutionary impact of selective brain cooling on the mammalian order Artiodactyla. PhD thesis, Ohio University, OhioLINK Electronic Theses and Dissertations Center.
- O'Leary MA. (2010) An anatomical and phylogenetic study of the osteology of the petrosal of extant and extinct artiodactylans (Mammalia) and relatives. Bull Am Mus Nat Hist 335: 1–206. [Google Scholar]
- Pross J, Bruch AA, Mosbrugger V, Kvacek Z (2001) Paleogene pollen and spores as a tool for quantitative paleoclimate reconstructions: the Rupelian (Oligocene) of central Europe. In Goodman DK, Clarke RT, eds, Proceedings of the IX International Palynological Congress, Houston, TX, USA, pp 299–310.
- Prothero DR. (1985) North American mammalian diversity and Eocene-Oligocene extinctions. Paleobiology 11: 389–405. [Google Scholar]
- Retallack G. (2007. a) Cenozoic paleoclimate on land in North America. J Geol 115: 271–294. [Google Scholar]
- Retallack G. (2007. b) Paleosols In Henke W, Tattersall I, eds, Handbook of Paleoanthropology, Vol 1, Principles, Methods and Approaches. Springer, Berlin, pp 383–408. [Google Scholar]
- Retallack GJ. (1991) Miocene Paleosols and Ape Habitats from Pakistan and Kenya. Oxford University Press, New York. [Google Scholar]
- Retallack GJ. (2001) Cenozoic expansion of grasslands and climatic cooling. J Geol 109: 407–426. [Google Scholar]
- Retallack GJ. (2013) Global cooling by grassland soils of the geological past and near future. Annu Rev Earth Planet Sci 41: 69–86. [Google Scholar]
- Ripple WJ, Newsome TM, Wolf C, Dirzo R, Everatt KT, Galetti M, Hayward MW, Kerley GIH, Levi T, Lindsey PA et al. (2015) Collapse of the world's largest herbivores. Sci Adv 1: e1400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroter RC, Robertshaw D, Zine Filali R (1989) Brain cooling and respiratory heat exchange in camels during rest and exercise. Respir Physiol 78: 95–105. [DOI] [PubMed] [Google Scholar]
- Schummer A, Wilkens H, Vollmerhaus B, Habermehl K-H (1981) The Anatomy of the Domestic Animals, Vol 2, The Circulatory System, the Skin, and the Cutaneous Organs of the Domestic Mammals. Paul Parey Press, Berlin. [Google Scholar]
- Sisson S, Grossman JD (1967) The Anatomy of the Domestic Animals. W.B. Saunders, Philadelphia. [Google Scholar]
- Smiles KA, Elizondo RS, Barney CC (1976) Sweating responses during changes of hypothalamic temperature in the rhesus monkey. J Appl Physiol 40: 653–657. [DOI] [PubMed] [Google Scholar]
- Spaulding M, O'Leary MA, Gatesy J (2009) Relationships of Cetacea (Artiodactyla) among mammals: increased taxon sampling alters interpretations of key fossils and character evolution. PLoS One 4: e7062 doi:7010.1371/journal.pone.0007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt JT. (1976) The regulation of respiratory evaporative heat loss in the rabbit. J Physiol 258: 157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss WM, Hetem RS, Mitchell D, Maloney SK, Meyer LCR, Fuller A (2015) Selective brain cooling reduces water turnover in dehydrated sheep. PLoS One 10: e0115514 doi:0115510.0111371/journal.pone.0115514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss WM, Hetem RS, Mitchell D, Maloney SK, Meyer LCR, Fuller A (2016) Three African antelope species with varying water dependencies exhibit similar selective brain cooling. J Comp Physiol B 186: 527–540. [DOI] [PubMed] [Google Scholar]
- Taylor C, Lyman C (1972) Heat storage in running antelopes: independence of brain and body temperatures. Am J Physiol 222: 114–117. [DOI] [PubMed] [Google Scholar]
- Vesterdorf K, Blache D, Maloney SK (2011) The cranial arterio-venous temperature difference is related to respiratory evaporative heat loss in a panting species, the sheep (Ovis aries). J Comp Physiol B 181: 277–288. [DOI] [PubMed] [Google Scholar]
- Webb SD, Taylor BE (1980) The phylogeny of hornless ruminants and a description of the cranium of Archaeomeryx. Bull Am Mus Nat Hist 167: 117–158. [Google Scholar]
- Wible JR. (1984) The ontogeny and phylogeny of the mammalian cranial arterial pattern. PhD thesis, Duke University, North Carolina, USA.
- Willmer P, Stone G, Johnston I (2009) Environmental Physiology of Animals. Wiley-Blackwell, Oxford, UK. [Google Scholar]
- Wing SL. (1987) Eocene and Oligocene floras and vegetation of the Rocky Mountains. Ann Missouri Bot Gard 74: 748–784. [Google Scholar]
- Withers PC, Cooper CE, Maloney SK, Bozinovic F, Cruz-Neto AP (2016) Ecological and Environmental Physiology of Mammals. Oxford University Press, New York. [Google Scholar]
- Wolfe JA. (1978) A paleobotanical interpretation of tertiary climates in the northern hemisphere: data from fossil plants make it possible to reconstruct Tertiary climatic changes, which may be correlated with changes in the inclination of the earth's rotational axis. Am Sci 66: 694–703. [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292: 686–693. [DOI] [PubMed] [Google Scholar]
- Zoological Society of London (2014) EDGE. http://edgeofexistence.org/