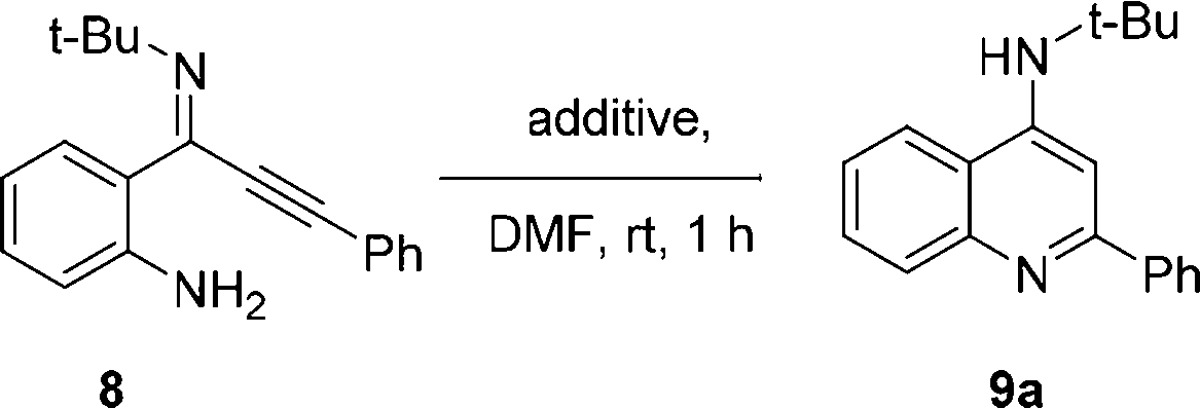
Table 1. Ynimine Cyclization Optimization.
| entrya | additive (equiv) | yield of 9 (%)b |
|---|---|---|
| 1 | NaOEt (2.0) | nr |
| 2 | HNEt3 (2.0) | nr |
| 3c | SiO2 | trace |
| 4 | AcOH (4.0) | trace |
| 5 | HCl in H2O (4.0) | 100 |
| 6 | HCl in MeOH (4.0) | 96 |
| 7 | HCl in dioxane (4.0) | 99 |
| 8 | TFA (4.0) | 80 |
| 9 | PhP(OH)2 (4.0) | 85 |
| 10 | MeSO3H (4.0) | 100 |
Conditions: ynimine 8 (0.55 mmol) in DMF (1 mL), additive, rt, 1 h.
Yield determined by NMR using an internal standard (1,3,5-trimethoxybenzene).
Using 0.150 g of SiO2; nr = no reaction, TFA = trifluoroacetic acid.