Table 2. Optimization of the Imidoylative Sonogashira/Cyclization Conditionsa.
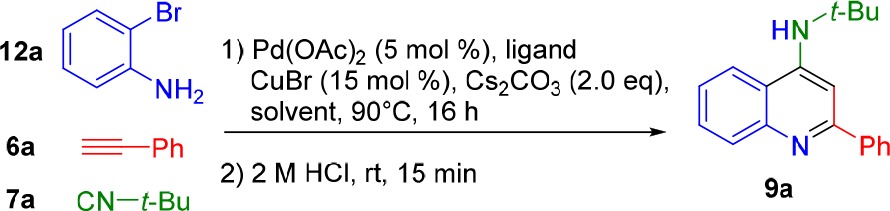
| entry | Pd source | ligand | solvent | 9a (%)b |
|---|---|---|---|---|
| 1c | Pd(PPh3)4 | none | DMSO | nr |
| 2 | Pd(PPh3)4 | none | DMSO | 61 |
| 3 | Pd(PPh3)4 | none | toluene | 27 |
| 4 | Pd(PPh3)4 | none | dioxane | 13 |
| 5 | Pd(PPh3)4 | none | DMF | 70 |
| 6 | Pd/C | none | DMF | nr |
| 7 | Pd(OAc)2 | PPh3 | DMF | 82 |
| 8 | Pd(OAc)2 | Bu3P | DMF | nr |
| 9 | Pd(OAc)2 | DPEPhos | DMF | 82 |
| 10 | Pd(OAc)2 | XantPhos | DMF | 91 |
| 11d | Pd(OAc)2 | XantPhos | DMF | nr |
| 12e | Pd(OAc)2 | XantPhos | DMF | 56 |
| 13f | Pd(OAc)2 | XantPhos | DMF | nr |
| 14g | Pd(OAc)2 | XantPhos | DMF | 31 |
| 15 | Pd(OAc)2 | none | DMF | nr |
| 16 | none | XantPhos | DMF | nr |
Reaction conditions: 2-bromoaniline (12a, 0.5 mmol, 1 equiv), phenylacetylene (6a, 1 mmol, 2 equiv), tert-butyl isocyanide (7a, 0.625 mmol, 1.25 equiv), catalyst (5 mol %), ligand (monodentate: 15 mol %, bidentate: 10 mol %), CuBr (15 mol %), and Cs2CO3 (1 mmol, 2 equiv) in solvent (3.0 mL) were stirred at 100 °C for 16–20 h under N2 atmosphere.
Yields determined by 1H NMR analysis using 2,5-dimethylfuran as internal standard.
Reaction performed in the absence of CuBr.
KOtBu (2.0 equiv) employed as base.
K2CO3 (2.0 equiv) employed as base.
Et3N (2.0 equiv) employed as base.
DBU (2.0 equiv) employed as base.
