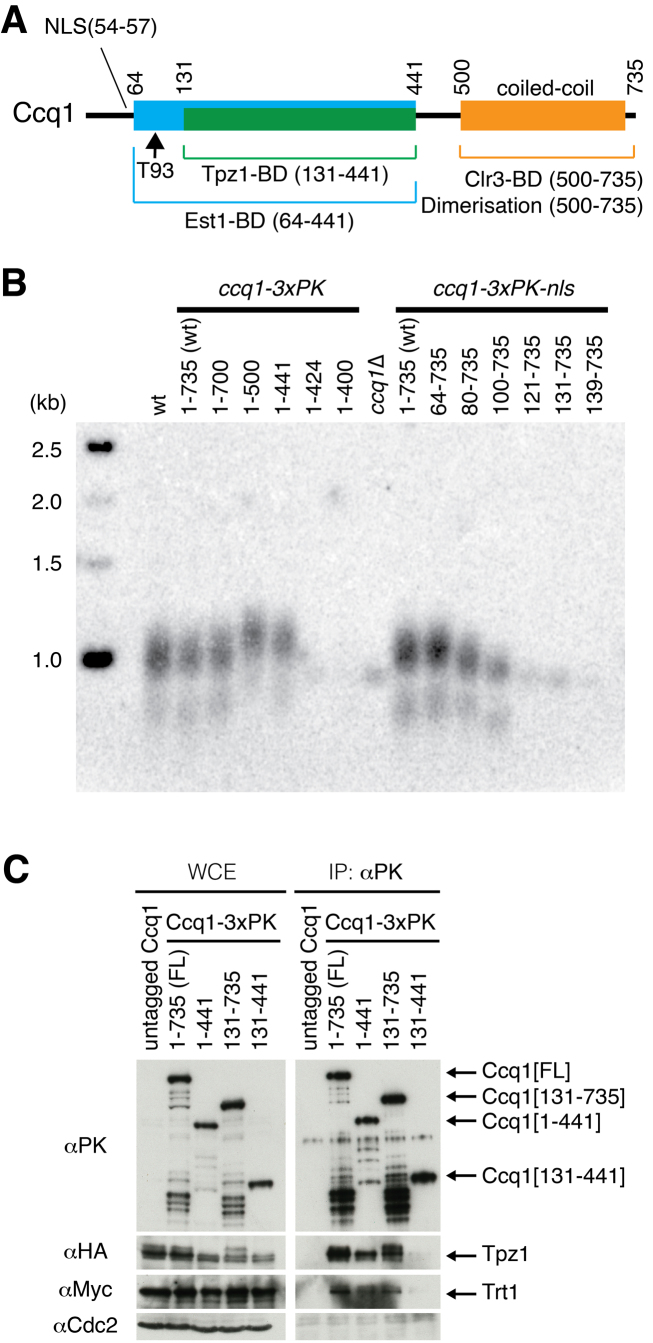
Figure 1.
Ccq1 C-terminus negatively controls telomere lengthening. (A) Schematic diagram of Ccq1 domains. The protein interaction regions were primarily assessed by the yeast two hybrid system. The core domain of Ccq1 stems from the region 131–441 residues that interacts with Tpz1 and is essential for any function of Ccq1. The Est1 binding domain contains residues including Threonine 93 and the Tpz1-binding domain and therefore begins from 64th amino acids and includes the Tpz1 binding site. The Ccq1 dimerization and Clr3 binding domain is located on the C-terminus (amino acids 500–735), which includes the coiled-coil motifs. (B). Telomere Southern blot shows that truncation of the coiled-coil motifs in Ccq1 leads to slight elongation of telomeres. The ccq1(1–500) and (1–441) truncation mutants exhibited slightly longer telomeres, but further C-terminal truncation or truncation of the Est1 binding domain resulted in maintenance of short telomeres, equivalent to ccq1Δ. EcoRI digested telomere fragments are an average length of 1 kb in wild type cells. (C) Co-immunoprecipitation of the PK epitope-tagged Ccq1 truncations revealed association with Tpz1 and Trt1. Although the Ccq1(131–441) truncation interacted with Tpz1 in the yeast two-hybrid system (Supplementary Figure S2A), neither this interaction nor telomerase association could be detected by co-immunoprecipitation. Slower migrating bands of Tpz1 are presumably caused by phospho-modifications, which are lost in ccq1(1–441) and ccq1(131–441) backgrounds.