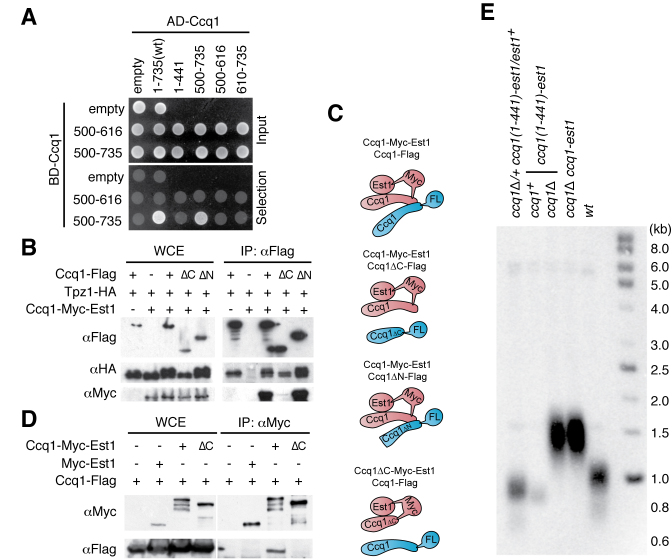
Figure 4.
The coiled-coil motifs region of Ccq1 forms a homodimer/multimer. (A) The yeast two-hybrid assay shows that the C-terminal coiled-coil motifs (500–735) forms a dimer/multimer. The indicated Ccq1 truncation proteins were fused to the GAL4 activation domain (AD) and the C-terminus fragments of Ccq1 were fused to the GAL4 DNA binding domain (BD). Selection plate lacks adenine and histidine. (B) The Ccq1-Est1 chimera and Tpz1 were co-immunoprecipitated with the FLAG epitope-tagged truncated Ccq1 (ΔC: 1–441aa; ΔN: 131–735aa). While Tpz1 interacted with both Ccq1 truncations, the C-terminus truncation failed to interact with the Ccq1-Est1 chimera. (C) Summary representation of co-immunoprecipitation study in B and D. Ccq1-Flag and Ccq1-Myc-Est1 interacts via their Ccq1 C-terminal domain. (D) Co-immunoprecipitation of the Myc epitope-tagged Est1 and the Ccq1-Est1 chimera (ΔC: Est1 is fused to Ccq1(1–441), the C-terminus truncation) showed that the Ccq1-Est1 chimera associated with Ccq1 via its C-terminus. (E) Telomere Southern blot shows that truncation of the Ccq1 C-terminus region within the Ccq1-Est1 chimera did not impair telomere lengthening. The elongation of telomeres by the Ccq1(1–441)-fused-Est1 chimera was similar to that by full length Ccq1-Est1 chimera in the absence of endogenous Ccq1.