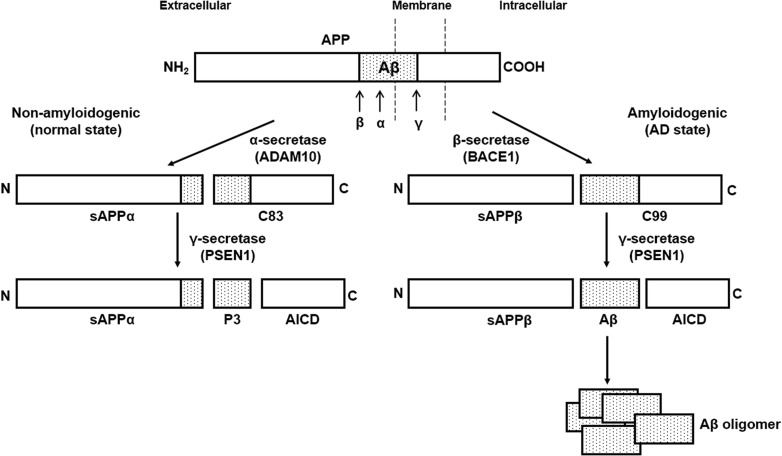
Fig. 1.
A simple diagram of APP processing and Aβ secretion in brain cells. The processing of amyloid precursor protein (APP) occurs in two distinct pathways: amyloidogenic and non-amyloidogenic. In the non-amyloidogenic pathway, APP is cleaved by α-secretase (ADAM10) at the site within the Aβ domain, releasing a soluble APPα (sAPPα) fragment and a C-terminal fragment (C83). The C83 fragment is further cleaved by γ-secretase (PSEN1) to release the APP intracellular domain (AICD) and a 3 kDa (p3) fragment. The amyloidogenic pathway involves the sequential cleavage of APP by β-secretase (BACE1) and γ-secretase (PSEN1). β-secretase generates a soluble APPβ (sAPPβ) fragment and a C-terminal fragment (C99), which is cleaved by γ-secretase (PSEN1) to release AICD and amyloid-β (Aβ) peptide. Secreted Aβ forms oligomers and aggregates, generating the toxic amyloid plaques observed in the AD patient’s brain (drawn by J. Park, and review by Hicks et al. [33]).