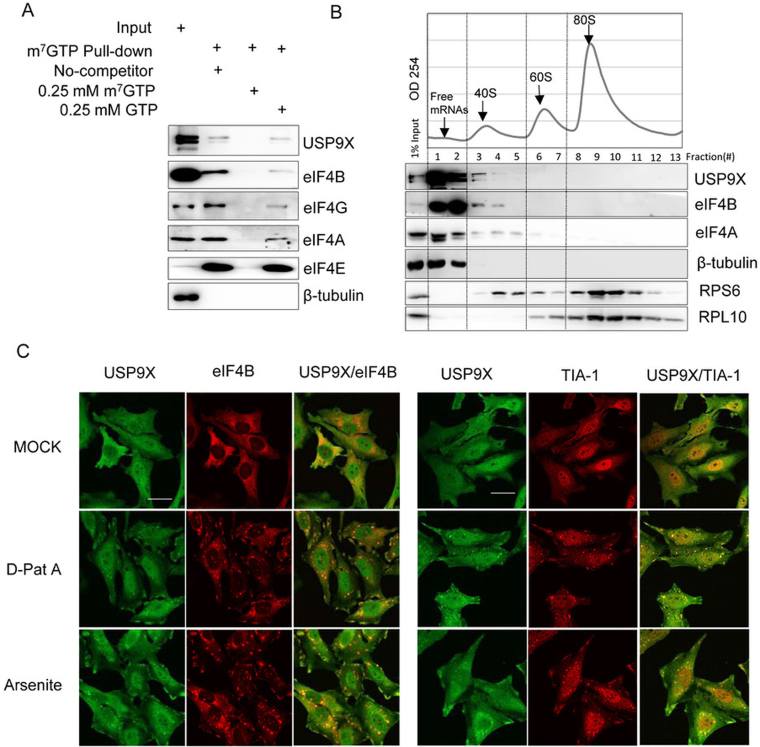
Figure 2.
USP9X co-localized and co-sedimented with the translation initiation complex. (A) HEK293T cell extracts were incubated with m7GTP-Sepharose beads in the presence or absence of 0.25 mM m7GTP or 0.25 mM GTP as indicated, and the eluted proteins were then analyzed using immunoblotting with the indicated antibodies. (B) Polyribosomal distribution of USP9X, eIF4B and eIF4A in HEK293T cells. Approximately 7 × 106 HEK239T cells were harvested and centrifuged over a 10%-35% sucrose gradient, and then RNA across the gradient were detected using a continuous 254-nm monitoring system as shown. The distribution of USP9X, eIF4B, eIF4A, β-Tubulin, RPS6 and RPL10 across the gradient was examined using immunoblotting. (C) Subcellular localization of USP9X upon either inhibition of translation initiation or oxidative stress. HeLa cells were treated upon with DMSO, 0.1 μM D-Pat A or 0.3 mM sodium arsenite for 1.5 h, and the localization of USP9X, eIF4B and the stress granule marker protein TIA-1 was monitored using immunofluorescence. Scale bar, 10 μm.