Abstract
Purpose:
The purpose of this study is to determine the tear and serum protein levels of interleukin-33 (IL-33) and its correlation with Th cytokines and disease severity in dry eye (DE) syndrome.
Methods:
Tear and serum samples were collected from 30 healthy volunteers, 30 DE patients with non-Sjogren's syndrome DE (NSSDE) and 30 DE patients with primary SSDE. The eight most frequent symptoms of DE were scored. All patients underwent corneal and conjunctival staining, tear film breakup time (TBUT), and Schirmer I test. The serum and tear levels of IL-33 were measured by enzyme-linked immunosorbent assay. The levels of IL-33 expression and its correlation with Th cytokines and disease severity were also analyzed.
Results:
We found that symptom scores and corneal staining grade were significantly higher in SSDE group compared with NSSDE and control group, whereas the results of TBUT and Schirmer I test were significantly lower in SSDE group compared with NSSDE and control group. The tear levels of IL-33 were significantly increased in tears of SSDE patients compared with those of controls and NSSDE patients (P < 0.05). Moreover, the elevated tear levels of IL-33 were positively correlated with symptom scores but negatively correlated with tear film breakup time and Schirmer I test in both NSSDE and SSDE patients (P < 0.05). The tear levels of IL-33 of both NSSDE and SSDE patients were also positively correlated with tear levels of IL-4 and IL-5 (P < 0.05). Correlation between the serum levels of IL-33 with Th1/17/Treg cytokines was not found.
Conclusion:
Elevated tear levels of IL-33 were associated with the Th2 cytokines and disease severity of DE. Therefore, IL-33 may have important roles in the immunopathogenesis of the DE.
Keywords: Dry eye, interleukin-33, Th cytokines
Dry eye (DE) is recently recognized as a multifactorial disease where loss of homeostasis of the tear film is the central pathophysiological characteristic.[1] DE diseases are often classified as aqueous deficient or evaporative DE. Tear-deficient DE can be mainly categorized as Sjogren's syndrome DE (SSDE) and non-SSDE (NSSDE). Artificial tears are often used in the treatment of DE. Moreover, inflammation in the pathogenesis of DE received more and more attention.[2,3,4,5] Therefore, anti-inflammatory drugs such as topical corticosteroids, tetracyclines, and topical cyclosporine A are prescribed.[6,7]
Interleukin-33 (IL-33), as a novel member of the IL-1 cytokine family, is broadly expressed by different tissues, such as smooth muscle cells, epithelial cells, fibroblasts, keratinocytes, dendritic cells, and activated macrophages.[8,9] The orphan IL-1 receptor, ST2, serves as the binding subunit for IL-33. The ST2 is expressed on mast cells, Th2 cells, and eosinophils and is necessary for induction of Th2 responses.[10] IL-33 has been implicated in asthma, allergic conjunctivitis, and anaphylactic responses.[11,12,13]
Th2 responses have recently been reported to play a role in Sjogren syndrome (SS), and Th2-associated cytokines were also detected in the tears of human DE subjects.[14,15] Besides, Th1 and Th17 responses were also involved in DE, whereas the role of Treg in DE was not clear.[15] Therefore, the aim of this study was to evaluate whether the IL-33 is involved in the pathogenesis in DE and its correlation with Th cytokines.
Methods
Study subjects
Sixty DE patients were recruited between 2016 and 2017 from our hospital. Primary SSDE and NSSDE were diagnosed clinically according to the criteria set by the International DE workshop report.[16] All enrolled patients presented with symptoms (dryness, sandy/gritty feeling, burning/stinging, sense of eye pressure, pain, sensitivity to light, and eye congestion) for at least 6 months and used only artificial tears that did not contain preservatives. All patients underwent tear film breakup time (TBUT), Schirmer I test, and corneal fluorescein staining in sequence. Each eye was examined separately at sufficient time intervals (more than 10 min). Besides, the interval between usage of artificial tears and any measurement was at least 2 h. Due to the unique female-to-male prevalence ratio in SS patients (Female: Male 9:1), we included only female patients in this study.
Exclusion criteria included the history of ocular surgery, contact lens use, and other ocular surface inflammation or infection. Patients who had morphologic changes in meibomian glands, such as vascular dilation, acinar atrophy, or orifice metaplasia, on the posterior lid margin were also excluded from our study. Patients on immune modulators and steroids at present or within 1 month were excluded. Thirty normal participants with comparable age and sex were those without ocular diseases or other basic diseases. This study was approved by the local Ethical Committee (No. 2015101001) and informed consent was obtained from each participant.
All the following examinations were done on each eye separately at sufficient time intervals (more than 10 min) to minimize the impact of having the same researcher conduct the experiments.
Symptom scores
Eight most frequent symptoms (eyestrain, dryness, sandy/gritty feeling, burning/stinging, sense of eye pressure, pain, sensitivity to light, and eye congestion) of DE were evaluated. Each symptom was scored from 0 to 9 with a maximum total score of 72.
Tear film breakup time test
Fluorescein strips wetted with 0.9% sodium chloride were gently applied to the inferior palpebral conjunctiva. After instillation of fluorescein and 3 or 4 times of blinking, the TBUT was measured using a slit lamp with maximum cobalt blue light. The patients were asked to open the eye widely and look straight ahead. The time before the appearance of defect in the stained tear film was recorded as the TBUT.
Corneal fluorescein staining
Corneal integrity was evaluated by fluorescein staining using a slit-lamp cobalt blue filter 1 min after a drop of 2% sterile fluorescein was instilled into the conjunctival sac. The Oxford grading scheme (score, 0–5) was adopted to evaluate the intensity of corneal staining.[17]
Schirmer I test
One sterile strip was placed in the lateral canthus of the inferior lid margin of both eyes without topical anesthesia. Participants closed their eyes during the test and the length of wetting was measured and recorded after 5 min.
Tear and serum collection
Around 50 μl of tear sample was collected as previously reported. Briefly, a microhematocrit tube (NRIS microhematocrit tube, Herlev, Denmark) was introduced into the inferior tear meniscus and tears were collected into the plastic tube using a bulb attached to one end of the tube. This process was duplicated two or three times at 4–6 min intervals. Venous blood samples were collected into Vacuette tubes and centrifuged at 3000 g for 15 min at 4°C. All samples were stored at –80°C until assay and tested within 2 h after thawing, followed by centrifugation. The samples were diluted 10-fold times before the test.
ELISA measurement for cytokines
ELISA kits were used for measuring serum IL-33, Th2 cytokines (IL-4, IL-5), Th1 cytokines (interferon [IFN]-gamma), Treg cytokines (IL-10, transforming growth factor-beta [TGF-β]), Th17 cytokines (IL-17), (R and D systems, SA) according to the manufacturer's protocols. The detection limits of the assays were as follows: IL-33, 1.65 pg/mL, IL-4, 1.56 pg/mL, IL-5, 7.8 pg/mL, IFN-gamma, 12.5 pg/mL, IL-10, 3.9 pg/mL, IL-17, 15 pg/mL, and TGF-β, 15.4 pg/mL.
Statistical analysis
Statistical analysis was performed using Mann–Whitney or Wilcoxon signed rank test. The pretest Friedman test was performed for multiple comparisons. The Spearman's rank correlation test and coefficient of determination were used to analyze the correlation between the expression of biomarkers. P < 0.05 was considered as significant difference.
Results
Demographic characteristics of study population and clinical outcome
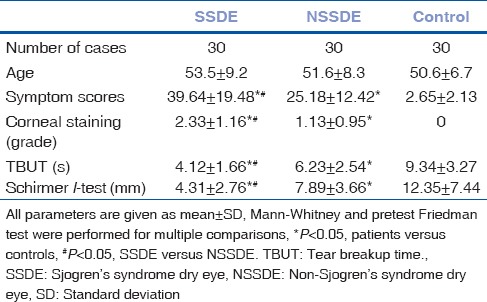
Thirty healthy volunteers, 30 patients with NSSDE, and 30 patients with SSDE were enrolled in this study. The demographic characteristics of patients were summarized in Table 1. Patients from three groups have comparable age. Symptom and fluorescein staining score were significantly higher in SSDE compared with NSSDE and control group (P < 0.05), whereas TBUT and Schirmer I were significantly lower in SSDE compared with NSSDE and control group (P < 0.05).
Table 1.
Demographics and clinical characteristics of study participants
Th1/Th2/Th17/treg cytokines expression in serum and tears
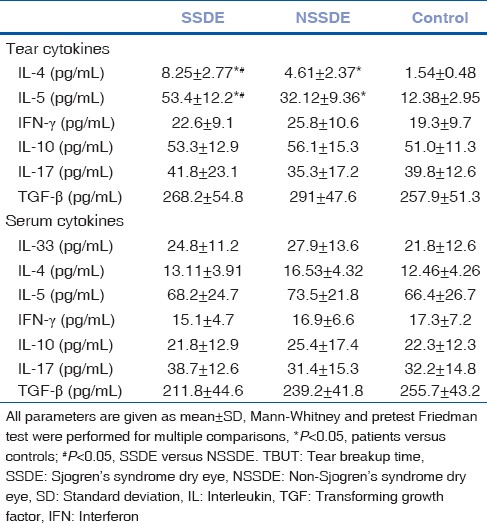
Tear IL-4 and IL-5 protein expression in SSDE patients increased significantly compared with controls and NSSDE patients [Table 2] (P < 0.05). However, the serum Th1/Th2/Th17/Treg and tear Th1/Th17/Treg cytokines expression between different groups showed no significant differences (Data not shown).
Table 2.
Levels of serum and tear cytokines in Sjogren syndrome dry eye patients, non-Sjogren syndrome dry eye patients, and normal participants
Elevated tear interleukin-33 protein levels in patients and its relationship with Th cytokines and disease severity
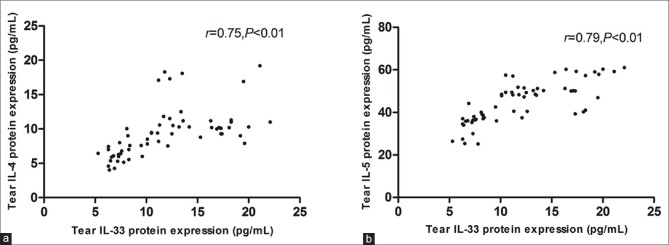
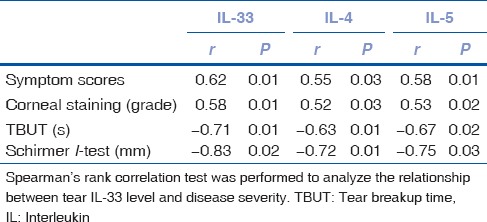
The tear IL-33 protein expression in SSDE patients increased significantly compared with controls and NSSDE patients [Fig. 1]. Correlation analysis showed that the tear IL-33 expression in SSDE and NSSDE patients was positively correlated to Th2 cytokines [Fig. 2]. When the coefficient of determination was introduced, the correlation between IL-33 and Th2 cytokines became weaker (IL-4, R2 = 0.56; IL-5, R2 = 0.62). Correlation between the serum levels of IL-33 with Th1/17/Treg cytokines was not found. Moreover, the elevated tear levels of IL-33 were positively correlated with symptom and fluorescein staining score but negatively correlated with tear film breakup time and Schirmer I test in both NSSDE and SSDE patients [Table 3].
Figure 1.
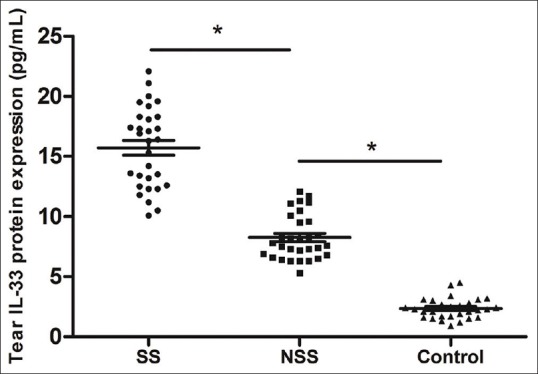
Tear interleukin-33 levels in tears Sjogren Syndrome dry eye patients and non-Sjogren Syndrome dry eye patients. Compared with non-Sjogren Syndrome group, tear interleukin-33 levels in SS group elevated significantly (P < 0.05). Compared with control group, tear interleukin-33 levels in non-Sjogren Syndrome group elevated significantly (P < 0.05)
Figure 2.
Correlation between interleukin-33 and Th2 cytokine levels in tears and clinical parameters in dry eye patients. Tear interleukin-33 expression in Sjogren's syndrome dry eye and non-Sjogren's syndrome dry eye patients was positively correlated to interleukin-4 (a) and interleukin-5 (b) respectively (r = 0.75, P < 0.01; r = 0.79, P < 0.01)
Table 3.
Relationship between tear interleukin-33 level and disease severity in dry eye patients
Discussion
Increasing evidence suggests that all forms of DE are characterized by varying ocular surface inflammation.[18] Previous studies had shown that the activation and infiltration of pathogenic immune cells, primarily CD4+ helper T cells in the conjunctiva and cornea, contribute to the inflammation in DE.[19] Consistently, elevated levels of inflammatory cytokines (IL-1, IL-6, TNF-alpha, and IL-17) were also found in the tear fluid and corneal and conjunctival epithelium.[15,20] However, the process and regulation of inflammation in DE was not clear. In this study, our data showed that elevated tear levels of IL-33 were associated with the Th2 cytokines and disease severity of DE, suggesting that IL-33 may have important roles in Th cell balance in the DE.
Our results showed that local Th2 inflammation was enhanced in both SSDE and NSSDE, especially in SSDE, whereas enhancement of system Th2 inflammation was not found in all groups. However, cytokines involved in the Th2 immune response, such as IL-5, were shown to be elevated in the salivary glands and serum in SS patients.[21] We also found that tear Th2 cytokines in SSDE were elevated compared with NSSDE patients, suggesting that SSDE contributes to more severe local Th2 inflammation. Interestingly, we did not found increased systemic Th2 response in DE compared with controls, which may be due to a small number of sample size or DE is just a local inflammation.
As for Th1/17/Treg inflammation, both local and system responses were not different among the three groups. SS was thought to be a Th1-dominant disease in the past.[22] In addition, Th-17, characterized by the secretion of inflammatory cytokines IL-17 and IL-23, was also shown to be involved in autoimmunity and inflammation in SS.[23] Our data suggests that Th1/17/Treg inflammation was not involved in DE inflammatory processes. Inconsistent with our results, Tan et al.[24] and Lee et al.[25]'s reports demonstrated enhanced Th17 inflammation in SS, whereas Boehm et al.[26]'s study suggested that Th1 response was dominant in SS. This discrepancy between our and other study regarding Th17 inflammation in DE may be due to different population or test methods.
IL-33 has the capacity to induce Th2 cytokine production from various immune cells, such as Th2 cells, mast cells, basophils, eosinophils, and newly identified innate immune cells, suggesting that IL-33 may be involved in Th2 cytokine-mediated allergic inflammation.[24,25,26] Indeed, IL-33 has been implicated in asthma, allergic conjunctivitis, and anaphylactic responses.[12,27] The previous studies had shown that the IL-33/ST2 axis was involved in the pathogenesis of SS and related to disease activity.[28,29] However, only serum IL-33 were measured in those studies and local IL-33 in tear was not analyzed. Our results showed that both tear IL-33 and Th2 cytokines expression in SSDE patients increased significantly compared with controls and NSSDE patients. Correlation analysis showed that tear IL-33 expression in SSDE and NSSDE was positively correlated to levels of Th2 cytokines, suggesting that tear IL-33 was involved in local Th2 inflammation. However, the correlation was not as strong as done in coefficient of correlation when coefficient of determination was introduced, suggesting that Th2 inflammation was influenced by multiple factors except for IL-33. The uncorrelated tear levels of IL-33 with Th1/17/Treg suggesting that IL-33 may have no direct effect on Th1/17/Treg regulation.
Moreover, the elevated tear levels of IL-33 and Th2 cytokines (IL-4/IL-5) were positively correlated with symptom scores but negatively correlated with tear film breakup time and Schirmer I test in both NSSDE. These results suggested that IL-33 may play important roles in the regulation of Th balance in the DE and thus lead to the progression of disease.
Conclusion
In sum, our results suggest that IL-33 may promote Th2 inflammation in the development of DE and contribute to disease severity. However, further studies with large samples and both in vivo and in vitro studies were needed to clarify the role of IL-33 and its interaction with the ST2 receptor in the pathogenesis of DE.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financaial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci. 2010;51:3083–91. doi: 10.1167/iovs.09-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC, et al. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205.e1. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enríquez-de-Salamanca A, Castellanos E, Stern ME, Fernández I, Carreño E, García-Vázquez C, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–73. [PMC free article] [PubMed] [Google Scholar]
- 5.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA, et al. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–7. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 6.Okada Y, Ikeda K, Yamanaka O, Miyamoto T, Kitano A, Kao WW, et al. TNFalpha suppression of corneal epithelium migration. Mol Vis. 2007;13:1428–35. [PubMed] [Google Scholar]
- 7.Kimura K, Teranishi S, Fukuda K, Kawamoto K, Nishida T. Delayed disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-alpha in a manner dependent on NF-kappaB. Invest Ophthalmol Vis Sci. 2008;49:565–71. doi: 10.1167/iovs.07-0419. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Sims JE, Nicklin MJ, Bazan JF, Barton JL, Busfield SJ, Ford JE, et al. A new nomenclature for IL-1-family genes. Trends Immunol. 2001;22:536–7. doi: 10.1016/s1471-4906(01)02040-3. [DOI] [PubMed] [Google Scholar]
- 10.Sims JE, Smith DE. The IL-1 family: Regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 11.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–30. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 12.Matsuba-Kitamura S, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Taki Y, Muto T, et al. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int Immunol. 2010;22:479–89. doi: 10.1093/intimm/dxq035. [DOI] [PubMed] [Google Scholar]
- 13.Pushparaj PN, Tay HK, H’ng SC, Pitman N, Xu D, McKenzie A, et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A. 2009;106:9773–8. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Pertovaara M, Antonen J, Hurme M. Th2 cytokine genotypes are associated with a milder form of primary Sjogren's syndrome. Ann Rheum Dis. 2006;65:666–70. doi: 10.1136/ard.2005.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pflugfelder SC, Corrales RM, de Paiva CS. T helper cytokines in dry eye disease. Exp Eye Res. 2013;117:118–25. doi: 10.1016/j.exer.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemp MA. Report of the national eye institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–32. [PubMed] [Google Scholar]
- 17.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Pflugfelder SC. Anti-inflammatory therapy of dry eye. Ocul Surf. 2003;1:31–6. doi: 10.1016/s1542-0124(12)70005-8. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol. 2009;2:375–6. doi: 10.1038/mi.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Volpe EA, Gandhi NB, Schaumburg CS, Siemasko KF, Pangelinan SB, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One. 2012;7:e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008;181:2898–906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 22.Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjögren's syndrome immunopathogenesis. Am J Pathol. 2009;175:1167–77. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roescher N, Tak PP, Illei GG. Cytokines in Sjogren's syndrome: Potential therapeutic targets. Ann Rheum Dis. 2010;69:945–8. doi: 10.1136/ard.2009.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan X, Sun S, Liu Y, Zhu T, Wang K, Ren T, et al. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye (Lond) 2014;28:608–13. doi: 10.1038/eye.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, et al. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients. Am J Ophthalmol. 2013;156:247–530. doi: 10.1016/j.ajo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011;52:7725–30. doi: 10.1167/iovs.11-7266. [DOI] [PubMed] [Google Scholar]
- 27.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–80. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 28.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 29.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]