Figure 1. Change in Plasma Levels of Proprotein Convertase Subtilisin–Kexin Type 9 (PCSK9), According to Study Group and Dose Cohort.
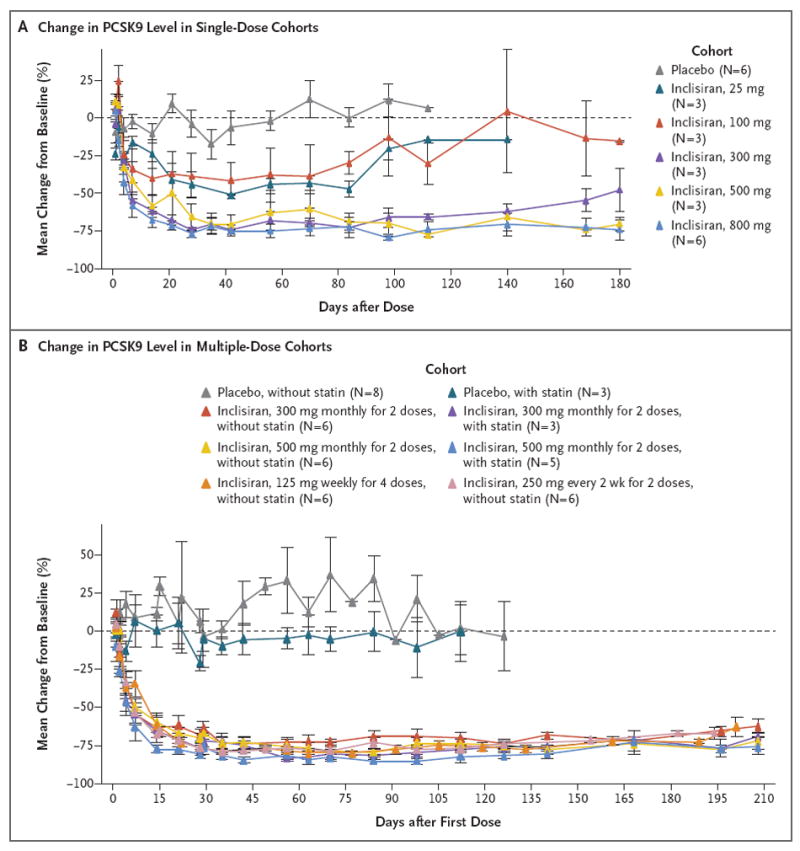
Shown are the effects (mean percentage changes from baseline) of single or multiple doses of inclisiran or placebo on plasma levels of PCSK9 over time. Baseline values were the average of all the study measurements obtained before the first dose. For the single-dose cohorts, the data for the placebo group include all the participants; for the multiple-dose cohorts, the data for the placebo group are presented according to whether the participants were or were not taking a stable baseline dose of statin cotherapy. I bars represent standard errors. For at least one cohort, only one value (for one participant) is shown at some time points. Participants were followed beyond the last planned visit only if the low-density lipoprotein cholesterol level had not returned to 80% of the baseline value by that time. In the single-dose phase, inclisiran or placebo was administered in one dose on day 0. In the multiple-dose phase, the first dose was administered on day 0 and subsequent doses at the indicated intervals. The 300-mg and 500-mg doses were administered as two monthly doses, the 125-mg dose was administered as four weekly doses, and the 250-mg dose was administered once every 2 weeks for 4 weeks.
